- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Alobresib (GS-5829) BET inhibitor
Cat.No.S8961
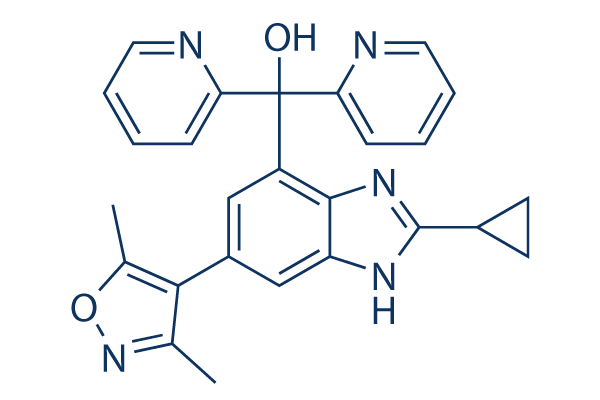
Chemical Structure
Molecular Weight: 437.49
Jump to
Quality Control
Solubility
|
In vitro |
DMSO
: 87 mg/mL
(198.86 mM)
Ethanol : 11 mg/mL Water : ˂1 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 437.49 | Formula | C26H23N5O2 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1637771-14-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=C(C(=NO1)C)C2=CC(=C3C(=C2)NC(=N3)C4CC4)C(C5=CC=CC=N5)(C6=CC=CC=N6)O | ||
Mechanism of Action
| Targets/IC50/Ki |
BET
BLK
Akt
ERK1/2
MYC
NF-κB
|
|---|---|
| In vitro |
In-vitro experiments demonstrates high sensitivity of USC cell-lines to the exposure to this compound causing a dose-dependent decrease in the phosphorylated levels of c-Myc and a dose-dependent increase in caspase activation (apoptosis). It inhibits CLL cell proliferation and induces leukemia cell apoptosis through deregulation of key signaling pathways, such as BLK, AKT, ERK1/2, and MYC. IκBα modulation indicates that this agent also inhibits NF-κB signaling. Its-induced apoptosis results from an imbalance between positive (BIM) and negative regulators (BCL-XL) of the intrinsic apoptosis pathway. |
| In vivo |
Alobresib (GS-5829) has impressive activity against USC primary tumors as well as USC xenografts. Assessment of c-Myc expression in tumors exposed to this compound demonstrates down-regulation of both total and phospho c-Myc proteins. This chemical demonstrates excellent bioavailability after oral administration and is significantly more effective than JQ1 at the doses used in comparative experiments in vivo against USC xenografts. Clinical studies with this compound in USC patients harboring disease resistant to standard salvage chemotherapy are warranted. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02983604 | Terminated | Advanced Estrogen Receptor Positive HER2- Breast Cancer |
Gilead Sciences |
January 10 2017 | Phase 1|Phase 2 |
| NCT02607228 | Terminated | Metastatic Castrate-Resistant Prostate Cancer |
Gilead Sciences |
December 8 2015 | Phase 1|Phase 2 |
| NCT02392611 | Completed | Solid Tumors and Lymphomas |
Gilead Sciences |
March 16 2015 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































