research use only
AZD5153 6-hydroxy-2-naphthoic acid BET inhibitor
Cat.No.S8344
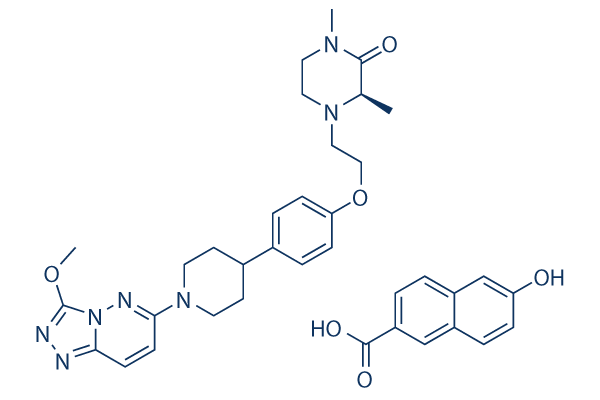
Chemical Structure
Molecular Weight: 667.75
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HCT116 | Function assay | 18 hrs | Displacement of Halo-tagged histone H3.3 from NanoLuc-tagged full length BRD4 (unknown origin) expressed in HCT116 cells after 18 hrs by NanoBRET assay , IC50 = 0.005 μM. | 28195723 | ||
| HCT116 | Function assay | 18 hrs | Displacement of Halo-tagged histone H3.3 from N-terminal NanoLuc-tagged BRD4 bromodomain 1 (44 to 168 residues) (unknown origin) expressed in HCT116 cells after 18 hrs by NanoBRET assay , IC50 = 1.6 μM. | 28195723 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(149.75 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 667.75 | Formula | C25H33N7O3.C11H8O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1869912-40-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | AZD5153 HNT salt | Smiles | CC1C(=O)N(CCN1CCOC2=CC=C(C=C2)C3CCN(CC3)C4=NN5C(=NN=C5OC)C=C4)C.C1=CC2=C(C=CC(=C2)O)C=C1C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
FL-BRD4
(Cell-based assay) 5 nM
|
|---|---|
| In vitro |
Unlike previously described monovalent inhibitors, AZD5153 ligates two bromodomains in BRD4 simultaneously. AZD5153 treatment markedly affects transcriptional programs of MYC, E2F, and mTOR. Of note, mTOR pathway modulation is associated with cell line sensitivity to AZD5153. AZD5153 potently disrupts BRD4 foci in U2OS cells with an IC50 value of 1.7 nmol/L. AZD5153 efficiently downregulates MYC protein levels across the cell line panel irrespective of their sensitivity to AZD5153. AML, MM, and DLBCL cell lines are highly sensitive to AZD5153. |
| In vivo |
In vivo administration of AZD5153 leads to tumor stasis or regression in multiple xenograft models of acute myeloid leukemia, multiple myeloma, and diffuse large B-cell lymphoma. AZD5153 modulates MYC and HEXIM1 in AML xenograft tumors and human whole blood. AZD5153 is administered orally to mice bearing MV-4-11 xenografts, and pharmacodynamic activity (intratumoral levels of c-Myc) is measured at 2, 4, and 8 h postdose. A considerable decrease in c-Myc expression is observed out to 8 h post dose at free plasma levels of compound <0.2 μM. This decrease in c-Myc expression after treatment with AZD5153 is consistent with other published BET inhibitors. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | NRG1 / BRD4 / GAPDH NRG1 / BRD4 / GAPDH NRG1 / BRD4 / GAPDH BRD4 / C-MYC / GAPDH cle-PARP / cle-caspase3 / GAPDH Wee1 / CDK1 / p-CDK1 / GAPDH |
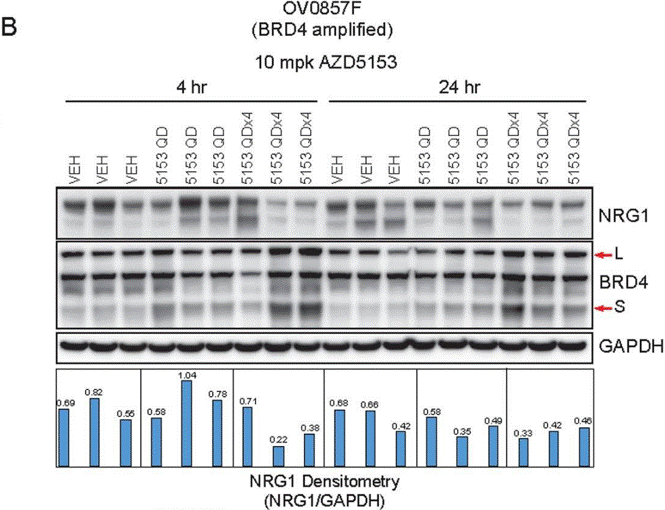
|
30036377 |
| Growth inhibition assay | Tumor Volume / Body Weight |
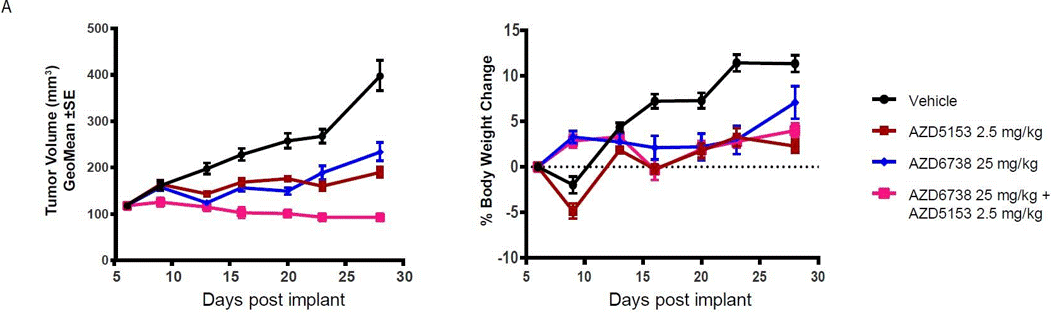
|
29636547 |
| IHC | cle-caspase3 / cle-PARP / Ki-67 |
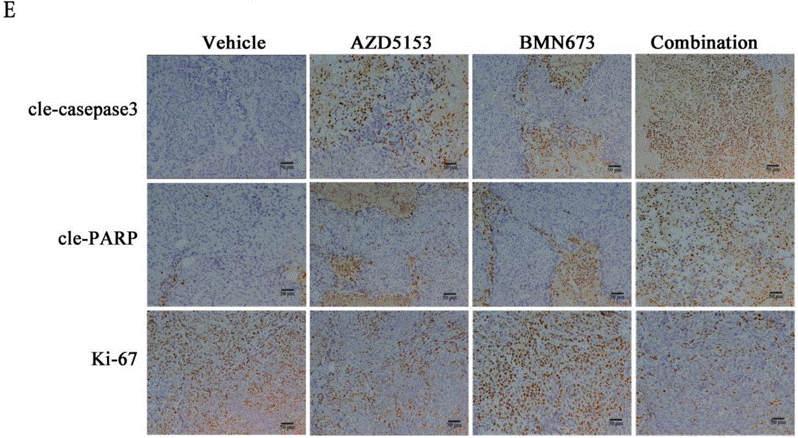
|
31523195 |
| Immunofluorescence | CDC6 p-S54 |
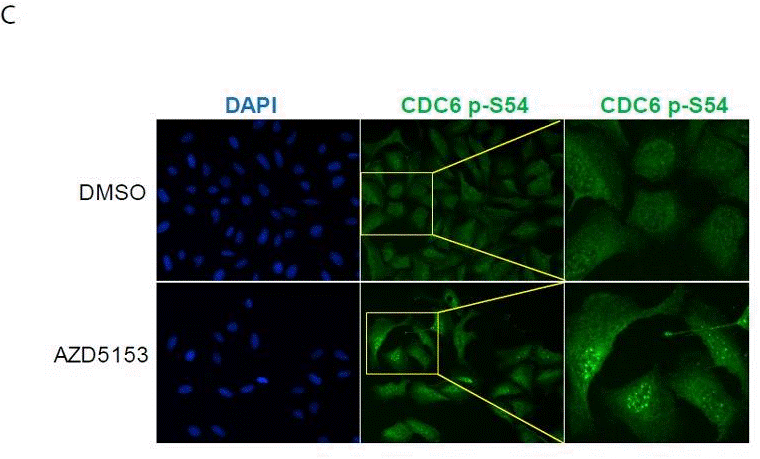
|
29636547 |
| ELISA | IL-10 / IL-12 |
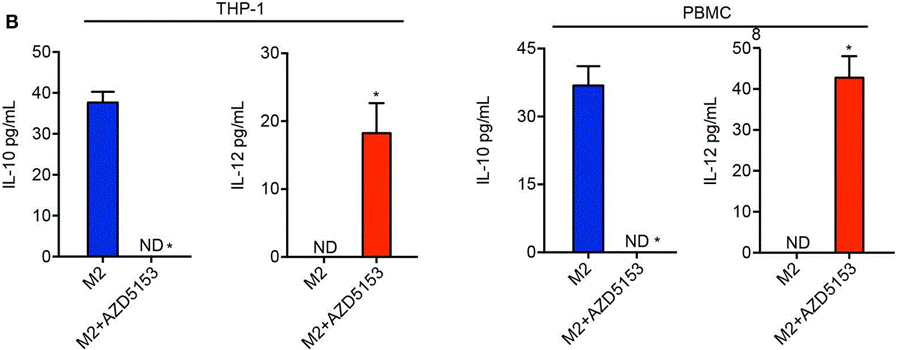
|
32184777 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03205176 | Completed | Malignant Solid Tumors|Lymphoma|Ovarian Cancer|Breast Cancer|Pancreatic Cancer|Prostate Cancer |
AstraZeneca |
June 30 2017 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































