research use only
Finasteride 5-alpha Reductase inhibitor
Cat.No.S1197
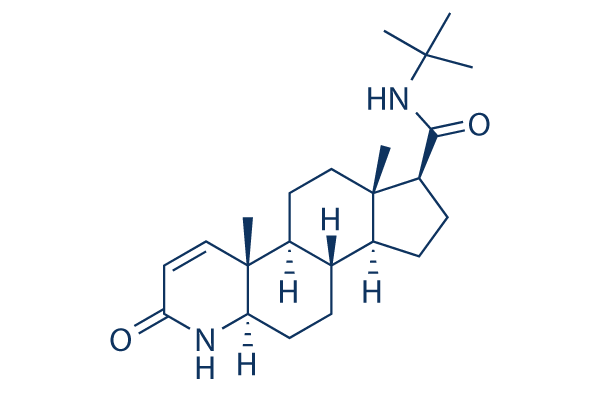
Chemical Structure
Molecular Weight: 372.54
Quality Control
Solubility
|
In vitro |
DMSO
: 75 mg/mL
(201.32 mM)
Ethanol : 18 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 372.54 | Formula | C23H36N2O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 98319-26-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | MK-906 | Smiles | CC12CCC3C(C1CCC2C(=O)NC(C)(C)C)CCC4C3(C=CC(=O)N4)C | ||
Mechanism of Action
| Targets/IC50/Ki |
5-α reductase
10.2 nM(Ki)
|
|---|---|
| In vitro |
Finasteride binds to the type 2 isozyme-NADPH complex to form a ternary complex with Ki of 1.19 nM, which then rearranges to a high affinity complex (E:I) with a pseudo first order rate constant of 1.62 ms. This compound dose-dependently inhibits the growth rate of the LnCap cell line. It markedly inhibits prostate-specific antigen (PSA) secretion and expression in LNCaP cells.
|
| In vivo |
Finasteride induces dosage-related incidences of hypospadias (penischisis) in male offspring with a threshold dosage level near 0.1 mg/kg/day and a 100% effect level of 100 mg/kg/day in male rats. This compound also causes decreased anogenital distance in male offspring in male rats. This chemical and castration decreases prostate weight at day 21 by 65% and 93%, respectively, in rats. It has no significant effect on DNA content after 4 days and decreases DNA content by a maximum of 52% at 14 days in rats. It causes a less intense increase in staining in which 16% of epithelial cells stained for tissue transglutaminase on day 9 with a return to baseline by day 14 in rats. This compound-induced staining is less intense with peak staining at day 4 (0.7% of epithelial cells) and a return to control values by day 9 in rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04288427 | Recruiting | Benign Prostatic Hyperplasia|Prostate Hyperplasia|Prostate Disease|Prostate Hypertrophy|Prostate Pain|Lower Urinary Tract Symptoms|Urinary Obstruction|Urinary Tract Disease |
Beth Israel Deaconess Medical Center|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) |
September 25 2020 | Not Applicable |
| NCT03669692 | Withdrawn | Prostatic Hyperplasia Benign|Metabolic Syndrome |
Complexo Hospitalario Universitario de A Coruña |
July 10 2018 | Not Applicable |
| NCT02824380 | Completed | Androgenic Alopecia |
Dong-A ST Co. Ltd. |
July 2016 | Phase 1 |
| NCT02146937 | Withdrawn | Detectable Prostate Nodules |
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins |
March 2014 | Phase 2 |
| NCT01703520 | Completed | Male Breast Cancer |
Organon and Co|Institute for Applied Economics and Health Research Aps |
May 1 2011 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































