- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
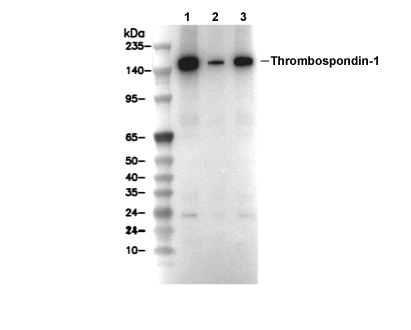
Thrombospondin 1 Antibody [N18C17]
Cat.No.: F4748
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP |
| Reactivity |
|---|
| Human, Mouse, Rat |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 170 kDa |
| Positive Control | ACHN cells; ScaBER cells; LN18 cells; mIMCD-3 cells; MEF cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| Thrombospondin 1 Antibody [N18C17] detects endogenous levels of total Thrombospondin 1 protein. |
| Clone |
|---|
| N18C17 |
| Synonym(s) |
|---|
| THBS1; Thrombospondin-1; Glycoprotein G; TSP; TSP1 |
| Background |
|---|
| Thrombospondin-1 (TSP-1 or THBS1) is a homotrimeric matricellular glycoprotein secreted by platelets, endothelial cells, and fibroblasts, and integrates into the extracellular matrix to regulate tissue remodeling through a wide array of ligand interactions. TSP-1 consists of an N-terminal globular TSPN domain that forms a β-sandwich of 13 antiparallel strands with a distinctive irregular β4′ segment and a disulfide bond at Cys248, enabling binding to heparin, aggrecan, and integrin αvβ3. This is followed by a von Willebrand factor C domain, three type I repeats (TSRs) characterized by stacked tryptophan/arginine ladders and conserved WXXWCSXG motifs that mediate engagement with CD36 and CD47 and inhibit MMP2/9, EGF-like type II repeats, and 15 calponin homology repeats with an RGD motif in the last repeat conferring affinity for integrins αvβ3 and αIIbβ3. The protein terminates with a C-terminal lectin-like globular domain that binds proteoglycans and stabilizes oligomers through interchain disulfide bonds at Cys252 and Cys256. TSP-1 potently inhibits angiogenesis by clustering CD47, thereby disrupting VEGF-R2 and nitric oxide (NO) signaling via SHP-2 phosphatase recruitment and blockade of eNOS S-nitrosylation. TSP-1 also activates latent TGF-β1 through integrin αvβ3/β8-mediated, shear-dependent unfolding of the latency-associated peptide, leading to Smad2/3 phosphorylation and fibrosis. It induces anoikis and CD36-mediated apoptosis in endothelial and smooth muscle cells via caspase-8/3 cascades and inhibition of Fyn/FAK, while also paradoxically promoting leukocyte transmigration and phagocytosis through calreticulin/LRP1/CD91-mediated engulfment of apoptotic bodies or pathogens. TSP-1 deficiency exacerbates tumorigenesis by permitting pathological angiogenesis and immune evasion in models of breast and prostate cancer, while its 3TSR fragment possesses strong anti-angiogenic activity when released by ADAMTS1 cleavage. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.