- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
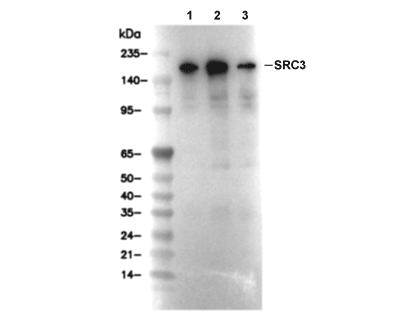
SRC3 Antibody [G12J12]
Cat.No.: F3882
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IHC, IF, FCM |
| Reactivity |
|---|
| Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 155 kDa |
| Positive Control | Human endometrium adenocarcinoma tissue; Daudi cells; HeLa cells; K562 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| SRC3 Antibody [G12J12] detects endogenous levels of total SRC3 protein. |
| Clone |
|---|
| G12J12 |
| Synonym(s) |
|---|
| AIB1; BHLHE42; RAC3; TRAM1; NCOA3; Nuclear receptor coactivator 3; NCoA-3; ACTR; Amplified in breast cancer 1 protein; AIB-1; pCIP; bHLHe42; RAC-3; SRC-3; TRAM-1 |
| Background |
|---|
| Steroid receptor coactivator-3 (SRC-3/AIB1/NCoA3), a p160 family member, functions as a master transcriptional coactivator that integrates growth factor signals to potentiate the activity of nuclear receptors (ERα, PR, AR, PPARγ) and non-receptor transcription factors (E2F1, AP-1, NF-κB, STAT3), controlling gene expression in development, metabolism, and oncogenesis. SRC-3 features an N-terminal bHLH/PAS domain for nuclear localization (via a bipartite NLS) and E2F1 binding, a central receptor interaction domain (RID) with three LXXLL motifs forming α-helices for ligand-dependent nuclear receptor binding, and C-terminal AD1/AD2 domains that recruit CBP/p300 (for H3/H4 acetylation via Q-rich motifs and intrinsic HAT activity) and CARM1/PRMT1 (for H3R17/26 methylation), as well as a polyglutamine tract. SRC-3 is regulated by post-translational modifications (PTMs), including Ser503/505 phosphorylation by AKT (for protein stability), Ser736 by CDK7/RSK (for coactivation), and Ser847 by IKK (for cytoplasmic-nuclear shuttling). Upon growth factor stimulation (EGF/IGF-1), MAPK/PI3K cascades phosphorylate SRC-3 in sequence, increasing LXXLL-nuclear receptor affinity and enabling FoxA1/ER intragenic looping for early gene activation. This leads to chromatin opening (via AD1 acetylation), Pol II pause release (via AD2 methylation), mTOR-driven polyribosome translation of oncogenic mRNAs, and 4E-BP1-mediated repression of pro-inflammatory cytokines in macrophages. In regulatory T cells, synergy with Foxp3 reprograms hundreds of genes to promote tumor immunosuppression. The PTM code orchestrates SRC-3 turnover (MAPK priming, AKT stabilization, SCF-mediated ubiquitin degradation). |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.