- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Sp7/Osterix Antibody [J12J9]
Cat.No.: F1545
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC |
| Reactivity |
|---|
| Mouse, Rat, Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
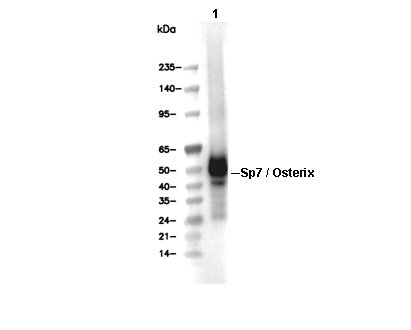
| Predicted MW Observed MW |
|---|
| 45 kDa 45 kDa, 47 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Human chondrosarcoma tissue; Mouse E14.5 rib tissue; Rat E14.5 rib tissue; Mouse E15 embryo; Rat E15 embryo; Mouse E14.5 developing humerus tissue; Saos-2 cell (1% SDS hot lysis); Saos-2 cell |
|---|---|
| Negative Control | Saos-2 cell (RIPA lysis) |
Exprimental Methods
| WB |
|---|
Experimental Protocol:
Sample Preparation
1. Tissue samples: Disrupt the tissue, add an appropriate amount of preheated Hot 1% SDS Lysis Buffer (containing Protease Inhibitor Cocktail), and homogenize at 90 - 95℃. 2. Adherent cell samples: Aspirate the culture medium and wash the cells twice with ice-cold PBS. Add an appropriate amount of preheated Hot 1% SDS Lysis Buffer (containing Protease Inhibitor Cocktail), perform thermal lysis at 90 - 95℃ for 10 minutes, and repeatedly pipette to resuspend the cells during this period to ensure full contact between the cells and the hot lysis buffer. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice.Add an appropriate amount of preheated Hot 1% SDS Lysis Buffer (containing Protease Inhibitor Cocktail), perform thermal lysis at 90 - 95℃ for 10 minutes, and repeatedly pipette to resuspend the cells during this period to ensure full contact between the cells and the hot lysis buffer. 4. Transfer the obtained homogenate/lysate to a centrifuge and centrifuge for 15 min, then collect the supernatant;
5. Take a small amount of the lysate to determine the protein concentration;
6. Add protein loading buffer, heat 20 μL of the sample at 95~100°C for 5 min, let it cool down on ice and then centrifuge for 5 min.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:1000), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
| IHC |
|---|
Experimental Protocol:
Deparaffinization/Rehydration
1. Deparaffinize/hydrate sections:
2. Incubate sections in three washes of xylene for 5 min each.
3. Incubate sections in two washes of 100% ethanol for 10 min each.
4. Incubate sections in two washes of 95% ethanol for 10 min each.
5. Wash sections two times in dH2O for 5 min each.
6.Antigen retrieval: For Citrate: Heat slides in a microwave submersed in 1X citrate unmasking solution until boiling is initiated; continue with 10 min at a sub-boiling temperature (95°-98°C). Cool slides on bench top for 30 min.
Staining
1. Wash sections in dH2O three times for 5 min each.
2. Incubate sections in 3% hydrogen peroxide for 10 min.
3. Wash sections in dH2O two times for 5 min each.
4. Wash sections in wash buffer for 5 min.
5. Block each section with 100–400 µl of blocking solution for 1 hr at room temperature.
6. Remove blocking solution and add 100–400 µl primary antibody diluent in to each section. Incubate overnight at 4°C.
7. Remove antibody solution and wash sections with wash buffer three times for 5 min each.
8. Cover section with 1–3 drops HRPas needed. Incubate in a humidified chamber for 30 min at room temperature.
9. Wash sections three times with wash buffer for 5 min each.
10. Add DAB Chromogen Concentrate to DAB Diluent and mix well before use.
11. Apply 100–400 µl DAB to each section and monitor closely. 1–10 min generally provides an acceptable staining intensity.
12. Immerse slides in dH2O.
13. If desired, counterstain sections with hematoxylin.
14. Wash sections in dH2O two times for 5 min each.
15. Dehydrate sections: Incubate sections in 95% ethanol two times for 10 sec each; Repeat in 100% ethanol, incubating sections two times for 10 sec each; Repeat in xylene, incubating sections two times for 10 sec each.
16. Mount sections with coverslips and mounting medium.
|
Biological Description
| Specificity |
|---|
| Sp7/Osterix Antibody [J12J9] detects endogenous levels of total Sp7/Osterix protein. |
| Subcellular Location |
|---|
| Nucleus |
| Uniprot ID |
|---|
| Q8TDD2 |
| Clone |
|---|
| J12J9 |
| Synonym(s) |
|---|
| OSX; SP7; Transcription factor Sp7; Zinc finger protein osterix |
| Background |
|---|
| Sp7, also known as Osterix (Osx), is a zinc-finger transcription factor of the Sp/KLF family that functions as a master regulator of osteoblast differentiation downstream of Runx2, essential for bone formation in vertebrates and primarily expressed in osteoblasts, osteocytes, and chondrocytes. Sp7 contains a proline-rich N-terminal transactivation domain and a C-terminal DNA-binding domain with three C2H2-type zinc finger motifs highly homologous to Sp1/Sp3/Sp4, though amino acid variations in the zinc finger reduce affinity for canonical GC-boxes and enable interaction with AT-rich motifs via protein-protein contacts. Sp7 promotes mesenchymal progenitor commitment to mature osteoblasts by inducing genes like Col1a1, Bglap (osteocalcin), Ibsp (bone sialoprotein), Sparc (osteonectin), and Alp while suppressing chondrogenesis; it acts primarily as a co-activator in complexes with Dlx family homeodomain factors (e.g., Dlx5) that bind AT-rich homeodomain-response elements in osteoblast enhancers, driving synergistic transcriptional activation via the Sp7 N-terminus. This non-canonical mechanism supports osteocyte maturation, lacuno-canalicular network formation, bone matrix mineralization, and adult bone homeostasis through pathways like BMP, Wnt/β-catenin, and MAPK signaling. Mutations in SP7 cause osteogenesis imperfecta and osteoporosis by disrupting osteoblast differentiation and bone mass maintenance. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.