- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Progerin Antibody [J17D10]
Cat.No.: F2441
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB |
| Reactivity |
|---|
| Human |
| Source |
|---|
| Mouse Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
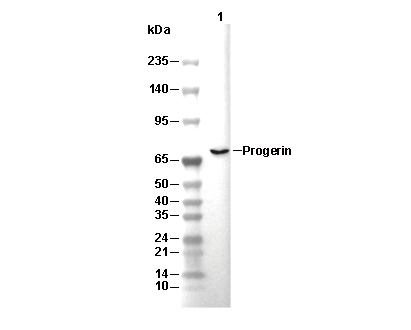
| Predicted MW Observed MW |
|---|
| 74 kDa 70 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
Biological Description
| Specificity |
|---|
| Progerin Antibody [J17D10] detects endogenous levels of total Progerin protein. |
| Clone |
|---|
| J17D10 |
| Synonym(s) |
|---|
| LMN1; LMNA; Prelamin-A/C |
| Background |
|---|
| Progerin (lamin AΔ50) is a pathogenic 607-amino-acid farnesylated truncation mutant of lamin A generated by a cryptic splice site activation in the LMNA gene (c.1824C>T, G608G), which eliminates the ZMPSTE24 cleavage site required for normal lamin A maturation. As a result, progerin retains a C-terminal CaaX (CSIM) farnesylation motif but lacks residues 647–656, preventing defarnesylation and leading to its permanent anchorage at the inner nuclear membrane. Progerin mirrors wild-type lamin A/C in its N-terminal head, central α-helical rod, and Ig-fold domains, but its aberrant farnesylation promotes toxic aggregate formation, clustering of SUN1/emerin, distortion of nuclear pore complexes, and increased lamina rigidity through enhanced F-actin coupling. Progerin disrupts nuclear architecture and genome maintenance by sequestering DNA repair factors (causing persistent 53BP1/γH2AX foci), depleting heterochromatin (H3K9me3 loss, lamina-associated domain relocation), and sustaining DNA damage signaling (ATM/ATR-Chk1/2-p53-p21 mediated senescence). It also impairs Wnt/β-catenin signaling, affects cytoskeletal-nuclear connections (deregulating ERK1/2 via abnormal LINC complexes), and drives cellular exhaustion and loss in vascular smooth muscle, adipocytes, and osteoblasts, manifesting clinically as the premature aging phenotypes of Hutchinson-Gilford Progeria Syndrome (HGPS), including lipodystrophy, osteolysis, and atherosclerosis. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.