- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
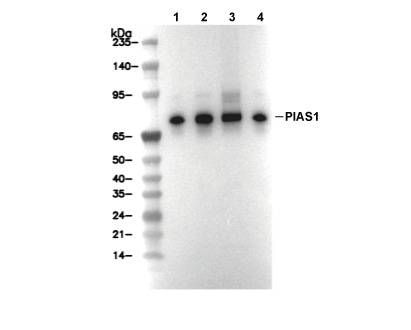
PIAS1 Antibody [J24P15]
Cat.No.: F4694
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IF, FCM |
| Reactivity |
|---|
| Human, Mouse, Rat, Monkey |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 76 kDa |
| Positive Control | RL cells; MOLT-4 cells; KARPAS-299 cells; NK-92 cells; SH-SY5Y cells; HeLa cells; AGS cells; SW480 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| PIAS1 Antibody [J24P15] detects endogenous levels of total PIAS1 protein. |
| Clone |
|---|
| J24P15 |
| Synonym(s) |
|---|
| PIAS1; E3 SUMO-protein ligase PIAS1; DEAD/H box-binding protein 1; E3 SUMO-protein transferase PIAS1; Gu-binding protein (GBP); Protein inhibitor of activated STAT protein 1; RNA helicase II-binding protein; DDXBP1 |
| Background |
|---|
| PIAS1 (protein inhibitor of activated STAT 1), a key E3 SUMO ligase and transcriptional corepressor of the PIAS family, negatively regulates JAK/STAT signaling by interacting with STAT1 to block its DNA-binding domain and promote SUMOylation at Lys703, featuring an N-terminal SAP domain forming a unique four-helix bundle for nuclear scaffold attachment and DNA targeting, a central SP-RING zinc-binding domain (RLD) coordinating Ubc9-dependent SUMO transfer via conserved Cys/His residues, a PINIT motif for nuclear retention, and a C-terminal region with a highly acidic domain (AD), serine/threonine-rich stretches, and SUMO-interaction motif (SIM) facilitating subnuclear relocalization of targets. PIAS1 dissociates from IKKα upon Ser90 phosphorylation by inflammatory cues to bind promoters and repress interferon-inducible genes like IFITM1, cooperates with HDACs/RCOR1/KDM1 to enforce transrepression independently of SUMOylation, inhibits NF-κB p65 and Smad-mediated transcription through direct binding and cofactor recruitment, modulates p53 activity by SUMOylation to alter subnuclear foci, and drives endothelial proliferation via S100A6 induction by suppressing STAT1, while PIAS1 knockout enhances antiviral protection yet promotes atherosclerosis through dysregulated KLF2/eNOS expression. PIAS1 overexpression sustains tumor progression by blocking STAT1 tumor-suppressive effects and enhancing angiogenesis, whereas its SUMO ligase activity counters viral replication (HSV-1 restriction via PML-NBs) but fosters drug resistance via STAT3/NF-κB crosstalk, with Arg303 methylation by PRMT1 fine-tuning repressive potency post-interferon stimulation. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.