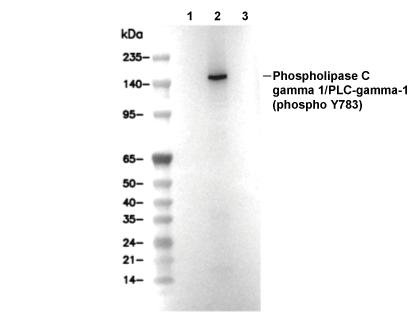
| Phospho-Phospholipase Cγ1 (Tyr783), the activated form of PLC-γ1, is a pivotal effector in receptor tyrosine kinase (RTK) signaling. Upon RTK stimulation, Tyr783 of PLC-γ1 becomes phosphorylated, typically by kinases such as EGFR, PDGFR, Syk, or Src family members. This phosphorylation event triggers a conformational change, relieving autoinhibition imposed by the split SH2-SH2-SH3 domains that otherwise clamp the catalytic core. The phosphorylated Tyr783 in the SH2 linker binds intramolecularly to the cSH2 domain, displacing it from the C2 core interface and exposing the active site for membrane docking and catalysis. PLC-γ1 features an N-terminal PH domain for PIP2 membrane recruitment, a catalytic TIM barrel (X-Y domains), EF hands and a C2 domain for Ca2+/lipid sensing, and regulatory SH2-SH2-SH3 domains. Once activated, PLC-γ1 hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). These second messengers drive intracellular calcium release and protein kinase C (PKC) activation, propagating downstream signaling. The high-affinity cSH2-pTyr783 interaction outcompetes autoinhibitory contacts, stabilizing an open, catalytically active conformation and amplifying IP3/DAG production. This sustains Ca2+ oscillations, PKC/ERK crosstalk, cytoskeletal remodeling, gene expression, and chemotaxis, especially in immune cells. Rapid dephosphorylation of Tyr783 ensures timely signal termination. Phospho-PLCγ1 is indispensable for platelet aggregation, T and B cell activation, and RTK-driven cell proliferation. Aberrant Tyr783 phosphorylation can cause immune disorders (e.g., SCID from gain-of-function mutations), hematologic malignancies via hyperactive PLC signaling, and drive invasion and angiogenesis in solid tumors. |