- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
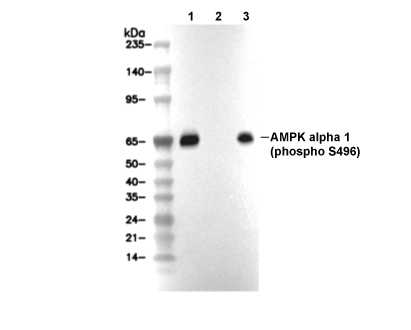
Phospho-AMPKα1 (Ser496) Antibody [K3H21]
Cat.No.: F3294
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IF |
| Reactivity |
|---|
| Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 64 kDa |
| Positive Control | 293T cells; HeLa cells |
|---|---|
| Negative Control | 293T cells (treated with LP) |
Biological Description
| Specificity |
|---|
| Phospho-AMPKα1 (Ser496) Antibody [K3H21] detects endogenous levels of total AMPKα1 protein only when it is phosphorylated at Ser496. |
| Clone |
|---|
| K3H21 |
| Synonym(s) |
|---|
| AMPK1; PRKAA1; 5'-AMP-activated protein kinase catalytic subunit alpha-1; AMPK subunit alpha-1; Acetyl-CoA carboxylase kinase; Hydroxymethylglutaryl-CoA reductase kinase; Tau-protein kinase PRKAA1; ACACA kinase; HMGCR kinase |
| Background |
|---|
| Phospho-AMPKα1 (Ser496) is an inhibitory phosphorylation event within the C-terminal regulatory domain of the α1 catalytic subunit of AMP-activated protein kinase (AMPK), a key heterotrimeric Ser/Thr kinase complex that senses cellular energy stress by monitoring AMP/ATP ratios. While activation of AMPK requires Thr172 phosphorylation in the activation loop, Ser496 phosphorylation occurs in a flexible autoinhibitory segment and is distinct from the kinase domain’s conserved architecture. This modification is primarily induced by PKA during adrenergic or cAMP-elevating conditions and by PKD or CKI during osmotic stress, and it sterically disrupts the heterotrimer assembly by weakening the interface between α and β subunits, blocking access of upstream kinases to Thr172, and thus attenuating AMPK activity even when energy depletion signals are present. Ser496 phosphorylation impairs nuclear translocation and reduces substrate engagement, leading to decreased phosphorylation of key targets like ACC, which dampens fatty acid oxidation. Mutations that prevent Ser496 phosphorylation enhance Thr172 phosphorylation and AMPK activity and improve insulin sensitivity under lipolytic stress, whereas persistent Ser496 phosphorylation contributes to sustained hyperglycemia in metabolic syndrome by blocking AMPK-mediated glucose uptake and autophagy. Ser496 phosphorylation helps tumor cells survive nutrient scarcity by suppressing cytostatic AMPK responses. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.