- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
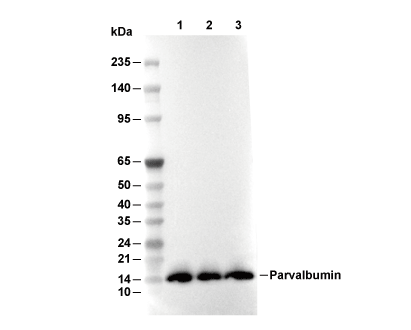
Parvalbumin Antibody [J22P8]
Cat.No.: F4591
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC, IF |
| Reactivity |
|---|
| Human, Mouse, Rat |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 12 kDa |
| Positive Control | Human cerebellum; Mouse cerebellum; Rat cerebellum; Mouse brain tissue; Human brain; Rat brain; Human kidney; Human B-cell non-Hodgkin lymphoma; Human parathyroid; Mouse colon; Renca syngeneic tumor; Rat eye; Mouse retina; RPMI 8226 cells |
|---|---|
| Negative Control | Mouse ovary; SK-MEL-28 cells |
Biological Description
| Specificity |
|---|
| Parvalbumin Antibody [J22P8] detects endogenous levels of total Parvalbumin protein. |
| Clone |
|---|
| J22P8 |
| Synonym(s) |
|---|
| Parvalbumin alpha; Alpha-parvalbumin; Alpha-PV; Pvalb; Pva |
| Background |
|---|
| Parvalbumin (PV, PVALB) is a small, acidic EF-hand calcium-binding protein highly expressed in fast-twitch skeletal muscle fibers and a specific subset of fast-spiking GABAergic interneurons in the brain, where it functions as a slow Ca2+ buffer that fine-tunes intracellular Ca2+ dynamics to support rapid muscle relaxation and high-frequency neuronal firing. PV is a compact protein comprising three helix-loop-helix EF-hand motifs—an AB domain (inactive due to a shortened DxDxDG loop) and functional high-affinity Ca2+/Mg2+-binding CD and EF domains with canonical 12-residue loops that coordinate Ca2+ via oxygen atoms from Asp, Glu, Asn, and main-chain carbonyls. These domains adopt distinct apo (open) and Ca2+-bound (closed) conformations, with the AB domain shielding the hydrophobic core and modulating CD/EF affinities. PV accelerates the initial decay phase of Ca2+ transients after action potentials without strongly reducing peak amplitude, thus preventing Ca2+ build-up, inhibiting short-term synaptic facilitation, and promoting rapid relaxation in muscle by shuttling Ca2+ from troponin C to the sarcoplasmic reticulum while minimizing twitch fusion during repetitive stimulation. In fast-spiking PV+ interneurons, this buffering supports perisomatic inhibition, gamma oscillations, and network synchrony essential for cognition. PV dysfunction or loss in PV+ neurons leads to circuit hyperexcitability, impaired working memory, and GABAergic deficits implicated in schizophrenia, autism spectrum disorders, Alzheimer’s disease, and epilepsy, while muscle-specific roles connect it to myopathies and malignant hyperthermia susceptibility. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.