- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
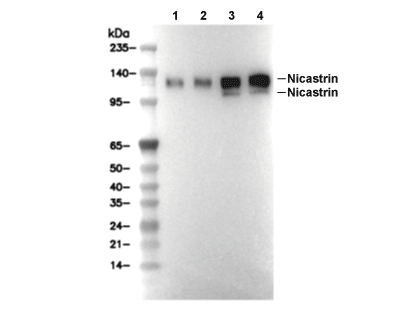
Nicastrin Antibody [H21H1]
Cat.No.: F0849
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IF |
| Reactivity |
|---|
| Human, Mouse, Rat, Monkey |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 110 kDa, 120 kDa |
| Positive Control | Mouse cerebellum; Mouse retina; Mouse liver; Mouse small intestine; HeLa cells; 3T3 cells; C6 cells; COS-7 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| Nicastrin Antibody [H21H1] detects endogenous levels of total Nicastrin protein. |
| Clone |
|---|
| H21H1 |
| Synonym(s) |
|---|
| NCSTN; Nicastrin; KIAA0253; UNQ1874/PRO4317 |
| Background |
|---|
| Nicastrin is an essential type I transmembrane glycoprotein and a core subunit of the γ-secretase complex, acting as the substrate receptor that recognizes and presents ectodomain-shed stubs of proteins like APP and Notch for intramembrane cleavage, which generates signaling peptides such as amyloid-β and the Notch intracellular domain implicated in Alzheimer's disease and developmental processes. It features a large, heavily glycosylated extracellular domain with a central β-sandwich core stabilized by disulfide bonds, a dynamic N-terminal lobe containing a substrate-binding pocket (including key residues in the DYIGS motif that capture free N-termini after ectodomain shedding), a single C-terminal transmembrane helix that interacts with presenilin, Aph1, and Pen2 to form the horseshoe-shaped γ-secretase complex, and a short cytoplasmic tail. The extracellular domain of nicastrin binds short hydrophilic N-terminal stubs of substrates through electrostatic interactions, positioning their transmembrane segments precisely above presenilin's catalytic aspartate residues for sequential γ-cleavage, while also stabilizing complex assembly and directing its trafficking to plasma and endosomal membranes. Mutations that disrupt the extracellular binding pocket can abolish substrate recognition without affecting complex formation. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.