- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
MUC1 Antibody [F23K8]
Cat.No.: F4884
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC, IF, FCM |
| Reactivity |
|---|
| Mouse, Rat, Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
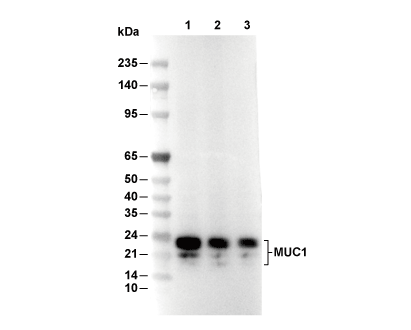
| Predicted MW Observed MW |
|---|
| 122 kDa 17-25 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Mouse lung tissue; Human colon cancer; Rat lung tissue; Normal stomach tissue; Human endometrium; Breast ductal infiltrating carcinoma tissue; Rat kidney; Mouse lung; A431 cells; MCF7 cells; T47-D cells; HeLa cells |
|---|---|
| Negative Control | Mouse liver tissue; Rat liver tissue |
Biological Description
| Specificity |
|---|
| MUC1 Antibody [F23K8] detects endogenous levels of total MUC1 protein. |
| Clone |
|---|
| F23K8 |
| Synonym(s) |
|---|
| CD227; PUM; MUC1; Mucin-1; MUC-1; Breast carcinoma-associated antigen DF3; Cancer antigen 15-3; Carcinoma-associated mucin; Episialin; H23AG; PEMT; Polymorphic epithelial mucin; CA 15-3; KL-6; PEM; EMA |
| Background |
|---|
| MUC1 is a transmembrane mucin glycoprotein that is overexpressed in the majority of epithelial carcinomas, functioning both as a protective barrier and a central oncogenic signaling hub. It undergoes autocatalytic cleavage at its SEA module, producing two non-covalently associated subunits: the extracellular, heavily O-glycosylated N-terminal (MUC1-N), which features extensive 20-amino-acid tandem repeats and extends hundreds of nanometers from the cell surface, and the membrane-anchored C-terminal (MUC1-C), which includes a short 72-amino-acid cytoplasmic tail rich in phosphorylation sites within the TFAHY motif. These phospho-acceptor sites are targeted by kinases such as Src, GSK3β, and PKCδ, enabling recruitment of β-catenin, EGFR, and Src, and supporting the nuclear translocation of MUC1-C. MUC1-C forms complexes that transactivate ErbB, Wnt, and inflammatory pathways, promoting cell proliferation, survival, epithelial-mesenchymal transition (EMT), invasion, and angiogenesis by upregulating genes like cyclin D1, c-Myc, Bcl-xL, vimentin, and VEGF. In tumors, hypoglycosylated MUC1 exposes peptide epitopes such as PDTRPAPGSTAPPAHGVT, which are recognized by immune T-cells but can also shield the tumor from immune surveillance. Truncation variants and mutations within the SEA module can impair MUC1 cleavage and signaling, contributing to the progression of cancers such as those of the breast, prostate, and pancreas. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.