- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
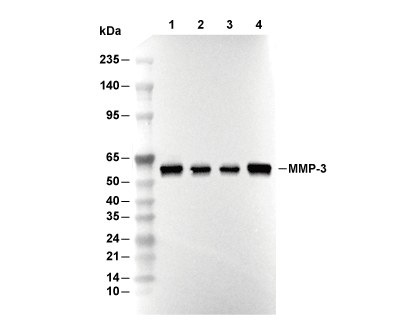
MMP-3 Antibody [K11K15]
Cat.No.: F4620
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB |
| Reactivity |
|---|
| Human, Rat |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 60 kDa |
| Positive Control | U-87 MG cells; U-118 MG cells; LN18 cells; PC-12 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| MMP-3 Antibody [K11K15] detects endogenous levels of total MMP-3 protein. |
| Clone |
|---|
| K11K15 |
| Synonym(s) |
|---|
| Stromelysin-1; SL-1; Matrix metalloproteinase-3 (MMP-3); Transin-1; MMP3; STMY1 |
| Background |
|---|
| Matrix metalloproteinase-3 (MMP-3), also known as stromelysin-1, is a zinc-dependent endopeptidase of the MMP family that degrades extracellular matrix components and is crucial for tissue remodeling during embryonic development, wound healing, and pathological processes such as cancer invasion and arthritis. MMP-3 has a modular structure featuring an N-terminal prodomain (~80 amino acids) that maintains latency via a cysteine switch with the catalytic zinc, a central catalytic domain with the conserved HExGHxxGxxH motif where three histidines coordinate Zn²⁺ for proteolysis, a hinge region, and a C-terminal hemopexin-like domain that facilitates substrate specificity. The hemopexin-like domain enables recognition of Phe residues in Gly-X-Phe motifs of triple-helical collagens through cooperative Cat-Hpx interactions, allowing efficient collagenolysis and a broad substrate repertoire including aggrecan, fibronectin, and laminin. MMP-3 is activated by proteolytic removal of the prodomain, often by furin or other MMPs, which unleashes its role in processes such as inflammation (by processing cytokines like IL-1β, chemokines, and growth factors), tumor progression (by cleaving E-cadherin to promote epithelial-mesenchymal transition and metastasis), and cardiovascular disease (through atherosclerotic plaque instability). Dysregulation of MMP-3 is linked to joint destruction in arthritis and cancer progression via upregulated transcription from AP-1/NF-κB sites. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.