- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
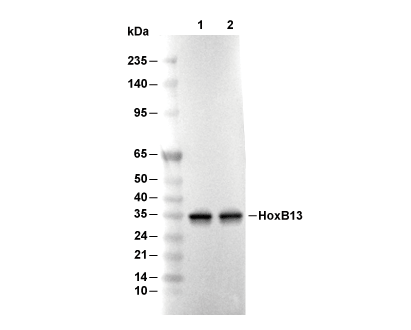
HoxB13 Antibody [E5D22]
Cat.No.: F4471
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC, IF, ELISA |
| Reactivity |
|---|
| Human |
| Source |
|---|
| Mouse Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 34 kDa |
| Positive Control | Human bladder; Human prostate; Human urinary bladder tissue; HeLa cells; LNCaP cells; PC-3 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| HoxB13 Antibody [E5D22] detects endogenous levels of total HoxB13 protein. |
| Clone |
|---|
| E5D22 |
| Synonym(s) |
|---|
| Homeobox protein Hox-B13; HOXB13 |
| Background |
|---|
| HOXB13 is a homeodomain-containing transcription factor from the HoxB cluster that directs posterior body patterning, prostate gland development, and urogenital tract morphogenesis by binding AT-rich DNA sequences with its homeodomain and recruiting cofactors such as MEIS and PBX to regulate chromatin accessibility and gene expression. The protein contains an N-terminal hexapeptide motif and PBX/MEIS-interacting domains that facilitate cooperative DNA binding and transactivation with PBX1/2/3, a central 60-residue homeodomain with three α-helices, including a recognition helix that inserts into the DNA major groove for TAAT-core specificity, and a C-terminal region where the G84 residue (the G84E mutation site) influences protein stability and repression of androgen receptor (AR) and TCF4 signaling. HOXB13–PBX1 heterodimers access gene enhancers to repress cell cycle genes such as CDC6 and MCM via HDAC3 recruitment and epigenetic silencing, while activating AR target genes like NKX3.1 through pioneer factor activity that remodels chromatin structure. HOXB13 modulates androgen signaling by inhibiting AR transcriptional activity at specific loci through direct binding interference and competition with FOXA1, but can also cooperate with AR to promote differentiation at other sites. Under oncogenic stress, the G84E mutation increases DNA binding affinity, disrupts MEIS cofactor interactions, and promotes AR-independent cell proliferation via β-catenin stabilization and epithelial-mesenchymal transition (EMT) through upregulation of ZEB1 and Snail. Clinically, germline G84E mutation substantially increases prostate cancer risk by altering cofactor dynamics and enhancer occupancy, somatic overexpression is associated with aggressive breast and ovarian cancers through metabolic reprogramming and lipogenesis suppression, and loss-of-function mutations cause hand-foot-genital syndrome due to defects in urogenital and lower limb development. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.