- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
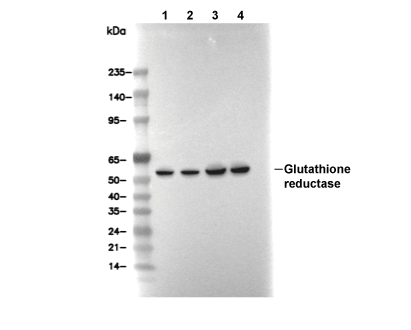
Glutathione reductase Antibody [E19C20]
Cat.No.: F4467
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC, IF, ELISA |
| Reactivity |
|---|
| Human, Mouse, Rat |
| Source |
|---|
| Mouse Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW |
|---|
| 50-65 kDa |
| Positive Control | Human fallopian tube tissue; Human parathyroid gland tissue; IMR-32 cells; JEG-3 cells; C6 cells; EOC 20 cells; H4 cells; SH-SY5Y cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| Glutathione reductase Antibody [E19C20] detects endogenous levels of total Glutathione reductase protein. |
| Clone |
|---|
| E19C20 |
| Synonym(s) |
|---|
| GSR; Glutathione reductase, mitochondrial; EC:1.8.1.7; GR; GRase; GLUR; GRD1 |
| Background |
|---|
| Glutathione reductase (GSR) is a dimeric flavoprotein enzyme belonging to the pyridine nucleotide-disulfide oxidoreductase family, essential for maintaining cellular redox homeostasis by catalyzing the NADPH-dependent reduction of oxidized glutathione (GSSG) to two molecules of reduced glutathione (GSH). GSH serves as the primary non-protein thiol antioxidant, detoxifying reactive oxygen species, peroxides via glutathione peroxidases, and xenobiotics through conjugation. Each monomer of glutathione reductase has an N-terminal FAD-binding Rossmann fold that holds the isoalloxazine ring, a central NADPH-binding domain with precise hydride-transfer geometry to position the nicotinamide ring close to the FAD, and a C-terminal dimerization/interface domain that forms the GSSG-binding pocket at the subunit interface. The enzyme possesses a redox-active disulfide (Cys58–Cys63 in humans) adjacent to FAD, where NADPH reduces FAD to FADH⁻ through a hydride transfer, generating a nucleophilic Cys58-thiolate that attacks the proximal sulfur of GSSG to form a mixed disulfide and release the first GSH. His467 then protonates the departing thiolate, and an intramolecular thiol-disulfide exchange with Cys63 regenerates the active disulfide, liberating the second GSH. This ping-pong bi-bi mechanism ensures high catalytic turnover and maintains a high GSH to GSSG ratio, which is crucial for defending against oxidative stress in the cytosol and mitochondria. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.