- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
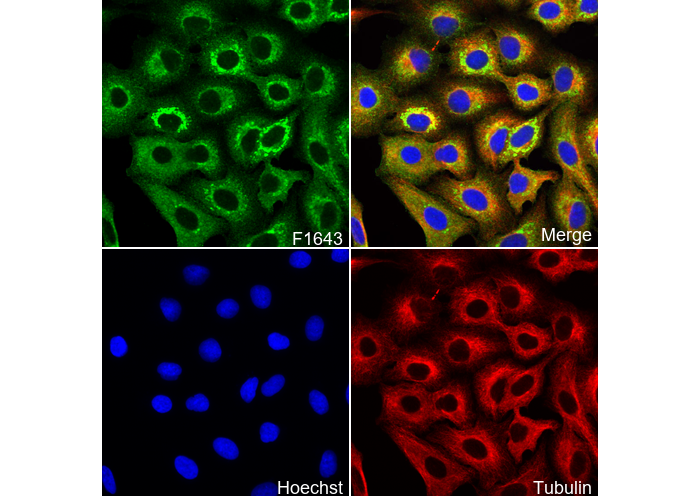
Glutathione Antibody [A11G7]
Cat.No.: F1643
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| IF, FCM |
| Reactivity |
|---|
| Source |
|---|
| Mouse Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Positive Control | Human monocytes; Human neutrophils; A549 cell (Apocynin, 10-500 μM, 24 h); RAW 264.7 cell |
|---|---|
| Negative Control |
Exprimental Methods
| IF |
|---|
Experimental Protocol:
Sample Preparation
1. Adherent Cells: Place a clean, sterile coverslip in a culture dish. Once the cells grow to near confluence as a monolayer, remove the coverslip for further use.
2. Suspension Cells: Seed the cells onto a clean, sterile slide coated with poly-L-lysine.
3. Frozen Sections: Allow the slide to thaw at room temperature. Wash it with pure water or PBS for 2 times, 3 minutes each time.
4. Paraffin Sections: Deparaffinization and rehydration. Wash the slide with pure water or PBS for 3 times, 3 minutes each time. Then perform antigen retrieval.
Fixation
1. Fix the cell coverslips/spots or tissue sections at room temperature using a fixative such as 4% paraformaldehyde (4% PFA) for 10-15 minutes.
2. Wash the sample with PBS for 3 times, 3 minutes each time.
Permeabilization
1.Add a detergent such as 0.1–0.3% Triton X-100 to the sample and incubate at room temperature for 10–20 minutes.
(Note: This step is only required for intracellular antigens. For antigens expressed on the cell membrane, this step is unnecessary.)
Wash the sample with PBS for 3 times, 3 minutes each time.
Blocking
Add blocking solution and incubate at room temperature for at least 1 hour. (Common blocking solutions include: serum from the same source as the secondary antibody, BSA, or goat serum.)
Note: Ensure the sample remains moist during and after the blocking step to prevent drying, which can lead to high background.
Immunofluorescence Staining (Day 1)
1. Remove the blocking solution and add the diluted primary antibody.
2. Incubate the sample in a humidified chamber at 4°C overnight.
Immunofluorescence Staining (Day 2)
1. Remove the primary antibody and wash with PBST for 3 times, 5 minutes each time.
2. Add the diluted fluorescent secondary antibody and incubate in the dark at 4°C for 1–2 hours.
3. Remove the secondary antibody and wash with PBST for 3 times, 5 minutes each time.
4. Add diluted DAPI and incubate at room temperature in the dark for 5–10 minutes.
5. Wash with PBST for 3 times, 5 minutes each time.
Mounting
1. Mount the sample with an anti-fade mounting medium.
2. Allow the slide to dry at room temperature overnight in the dark.
3. Store the slide in a slide storage box at 4°C, protected from light.
|
Biological Description
| Specificity |
|---|
| Glutathione Antibody [A11G7] detects endogenous levels of total Glutathione protein. |
| Subcellular Location |
|---|
| Cytoplasm, Mitochondrion |
| Uniprot ID |
|---|
| P00390 |
| Clone |
|---|
| A11G7 |
| Synonym(s) |
|---|
| GSH; Oxidised glutathione; Reduced glutathione |
| Background |
|---|
| Glutathione (GSH) is a low-molecular-weight tripeptide thiol antioxidant composed of glutamate, cysteine, and glycine, synthesized via two sequential ATP-dependent steps catalyzed by glutamate-cysteine ligase and glutathione synthetase. It primarily exists in reduced (GSH) and oxidized disulfide (GSSG) forms, which interconvert to regulate cellular redox balance. GSH features a unique γ-glutamyl peptide bond between glutamate’s γ-carboxyl and cysteine’s amino group, imparting resistance to degradation by γ-glutamyl cyclotransferase and most peptidases; the cysteine thiol (-SH) acts as the nucleophilic center for redox chemistry, while glycine stabilizes the C-terminus. GSH directly scavenges reactive oxygen and nitrogen species (ROS/RNS) via thiol-disulfide exchange, serves as an essential reductant for glutathione peroxidases (GPx) that detoxify peroxides (with GSSG reduced back to GSH by glutathione reductase using NADPH), and conjugates electrophilic xenobiotics, heavy metals, and endogenous toxins through glutathione S-transferases (GSTs) for phase II detoxification and mercapturic acid export. These processes maintain protein sulfhydryl homeostasis, support Nrf2-driven antioxidant gene expression, facilitate iron-sulfur cluster assembly and metal trafficking, and modulate signaling pathways such as NF-κB. GSH depletion is implicated in oxidative stress-related diseases, including Parkinson’s disease, liver cirrhosis, cancer, diabetes, and premature aging. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.