- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
FOXC1 Antibody [A1B22]
Cat.No.: F4830
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC, IF, FCM |
| Reactivity |
|---|
| Mouse, Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
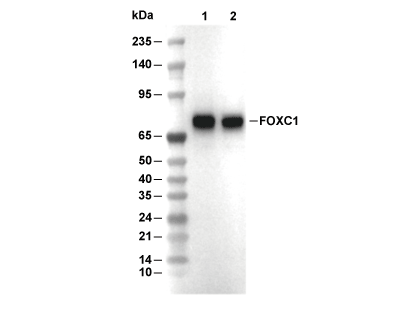
| Predicted MW Observed MW |
|---|
| 57 kDa 70 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Human basal-like breast cancer tissue; Human gastric cancer tissue; Mouse cerebrum tissue; Human fetal kidney; Mouse fetal brain E14.5 tissue; MDA-MB-231 cells; HEK-293T cells; HeLa cells |
|---|---|
| Negative Control | Jurkat cells |
Biological Description
| Specificity |
|---|
| FOXC1 Antibody [A1B22] detects endogenous levels of total FOXC1 protein. |
| Clone |
|---|
| A1B22 |
| Synonym(s) |
|---|
| FKHL7; FREAC3; FOXC1; Forkhead box protein C1; Forkhead-related protein FKHL7; Forkhead-related transcription factor 3; FREAC-3 |
| Background |
|---|
| FOXC1 is a Forkhead box transcription factor crucial for ocular, cardiovascular, and craniofacial development, binding the DNA consensus sequence GTAAACA through its winged-helix Forkhead domain (FHD), which comprises three α-helices, one of which acts as the recognition helix contacting the DNA major groove, two β-hairpin wing motifs, and a nuclear localization signal. The protein also includes an N-terminal activation domain, a central inhibitory or phosphorylation domain, and a C-terminal activation domain, together enabling context-dependent gene transactivation. FOXC1 activates genes involved in somitogenesis and myogenesis, such as MEOX1 and PAX3, as well as vascular smooth muscle markers by direct promoter binding, while repressing cell cycle progression through the induction of p21 and p27 and exclusion of β-catenin from the nucleus. Phosphorylation of serine/threonine residues within the inhibitory domain modulates the switch between transcriptional activation and repression, integrating signals from pathways like TGF-β/Smad. Mutations in the FHD that disrupt DNA binding or nuclear localization result in developmental disorders such as Axenfeld-Rieger syndrome, characterized by anterior segment dysgenesis, glaucoma, and iris hypoplasia. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.