- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
FGF2 Antibody [E17H24]
Cat.No.: F4828
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IF, FCM |
| Reactivity |
|---|
| Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
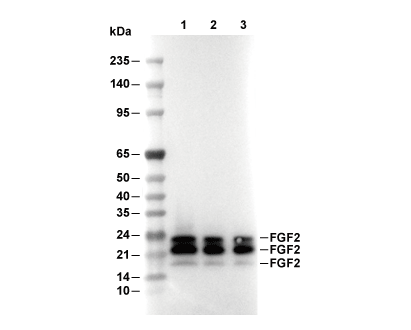
| Predicted MW Observed MW |
|---|
| 30 kDa 15-24 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Human fetal kidney; Human prostate; Human fetal heart; Human testis; SK-OV-3 cells; U-87 MG cells; K562 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| FGF2 Antibody [E17H24] detects endogenous levels of total FGF2 protein. |
| Clone |
|---|
| E17H24 |
| Synonym(s) |
|---|
| FGFB; FGF2; Fibroblast growth factor 2; FGF-2; Basic fibroblast growth factor; Heparin-binding growth factor 2; bFGF; HBGF-2 |
| Background |
|---|
| FGF2 (fibroblast growth factor 2, or basic FGF) is a prototypical heparin-binding member of the FGF family, secreted by various cell types, including endothelial cells, fibroblasts, and macrophages, to coordinate processes such as angiogenesis, wound healing, and development. FGF2 folds into a compact β-trefoil core composed of antiparallel β-strands linked by minimal loops, with an unstructured N-terminal segment outside the core and a C-terminal extension. Its primary receptor-binding sites are located on specific β-strands and the β4-β5 loop, with key residues mediating hydrogen bonding and hydrophobic contacts to the IgII domain of FGF receptors (FGFRs), and a secondary binding site in the β10-β12 region that engages both the IgIII domain and the linker between IgII and IgIII. A basic canyon formed by arginine and lysine residues coordinates heparan sulfate or heparin, stabilizing ternary signaling complexes. In canonical paracrine signaling, cell-surface heparan sulfate bridges FGF2-FGFR dimers in a symmetrical arrangement, with the FGF2 β-trefoil docking to FGFR’s D2 domain while the N-terminus and central β-strands interact with alternatively spliced D3 loops, conferring isoform specificity. FGFR activation through trans-autophosphorylation triggers downstream scaffolding of FRS2α-GRB2-SOS for the RAS-RAF-MEK-ERK signaling cascade, driving cell proliferation and differentiation; PLCγ-IP3 signaling for migration; and PI3K-AKT for survival and angiogenesis. Nuclear high-molecular-weight isoforms with C-terminal nuclear localization signals can directly transactivate genes such as CCND1 via ribosomal interaction, while low-molecular-weight FGF2 is exported independently of the ER/Golgi apparatus through a FGF2-heparan sulfate-FGFR1 import/export mechanism. Dysregulated FGF2 overexpression promotes tumor angiogenesis and microenvironment remodeling in cancers such as glioblastoma and hepatocellular carcinoma through VEGFR2 and PI3K pathway crosstalk and endothelial sprouting, mediates atherosclerotic plaque neovascularization via smooth muscle cell proliferation, and contributes to fibrosis and retinopathy. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.