- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
CaMKII-α Antibody [P6K24]
Cat.No.: F4909
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IHC |
| Reactivity |
|---|
| Mouse, Rat, Human |
| Source |
|---|
| Mouse Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
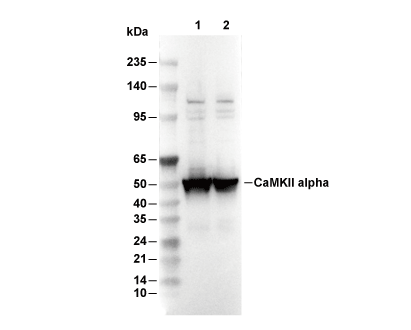
| Predicted MW Observed MW |
|---|
| 54 kDa 100 kDa, 50-70 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Human brain tissue; Rat brain tissue; Mouse brain tissue; Rat normal brain (Purkinje cells, cerebellum) |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| CaMKII-α Antibody [P6K24] detects endogenous levels of total CaMKII-α protein. |
| Clone |
|---|
| P6K24 |
| Synonym(s) |
|---|
| CAMKA; KIAA0968; CAMK2A; Calcium/calmodulin-dependent protein kinase type II subunit alpha; CaM kinase II subunit alpha; CaMK-II subunit alpha |
| Background |
|---|
| CaMKII-α (calcium/calmodulin-dependent protein kinase II alpha) is the principal neuronal isoform of the CaMKII serine/threonine kinase family and assembles into dodecameric or tetradecameric holoenzymes that are pivotal for synaptic plasticity and memory formation. CaMKII-α contains an N-terminal bilobal kinase domain (residues 1-270) where conserved Asp156 and Lys42 coordinate ATP in the catalytic cleft, an autoinhibitory regulatory domain (271-315) with a pseudosubstrate sequence in which Arg-to-Thr286 mimics substrate occlusion and blocks access until displaced by Ca²⁺/calmodulin (CaM), a variable linker region that allows for flexible kinase positioning, and a C-terminal hub/association domain (316-478) that forms a rigid toroidal β-sheet scaffold via four-helix bundles per subunit, supporting intersubunit allosteric interactions. Upon Ca²⁺/CaM binding, the regulatory domain is displaced, relieving autoinhibition and exposing Thr286 for intersubunit trans-autophosphorylation, which prevents CaM dissociation (the "CaM trap") and confers Ca²⁺-independent activity that can persist for over an hour. This mechanism enables CaMKII-α to decode Ca²⁺ oscillation frequency by sequential, wave-like phosphorylation that propagates bidirectionally around the holoenzyme ring, amplifying signaling events such as GluA1 Ser831 phosphorylation to drive AMPAR trafficking and long-term potentiation (LTP). Autophosphorylation at Thr305/306 or oxidation (Met281/282 sulfenic acid formation) further modulates autoinhibition recapture, while phosphatases PP1 and PP2A reverse Thr286 phosphorylation, a process that can be inhibited by PKA crosstalk. Pathologically, hyperactivity of α-CaMKII due to T286I mutation or SCN1A-linked disinhibition leads to epilepsy through hyperexcitable neuronal networks and dendritic defects; heterozygous knockout impairs hippocampal LTP and spatial memory, but can confer resilience to neurodegeneration. Additionally, isoform imbalance disrupts CaV1.2 channel coupling, contributing to cardiac arrhythmias. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.