- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Calsequestrin 1 Antibody [P19M13]
Cat.No.: F3992
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IHC |
| Reactivity |
|---|
| Mouse, Rat, Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
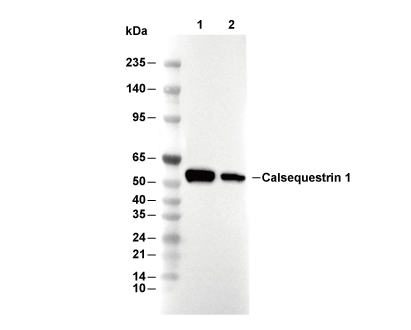
| Predicted MW Observed MW |
|---|
| 44 kDa 63 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Human skeletal muscle tissue; Rat muscle tissue; Mouse muscle tissue |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| Calsequestrin 1 Antibody [P19M13] detects endogenous levels of total Calsequestrin 1 protein. |
| Clone |
|---|
| P19M13 |
| Synonym(s) |
|---|
| CASQ, CASQ1, Calsequestrin-1, Calmitine |
| Background |
|---|
| Calsequestrin 1 (CASQ1) is the principal high-capacity, low-affinity Ca²⁺-binding protein in the sarcoplasmic reticulum (SR) terminal cisternae of fast-twitch skeletal muscle, crucial for enabling rapid Ca²⁺ release during muscle contraction while preventing calcium overload. CASQ1 is composed of three thioredoxin-like domains (I–III) formed by five-stranded β-sheets flanked by α-helices, and features flexible N- and C-terminal tails rich in aspartate and glutamate residues that create multiple Ca²⁺-binding pockets. At luminal Ca²⁺ concentrations around 1 mM, CASQ1 monomers polymerize through back-to-back C-terminal dimerization (mediated by Asp-rich CAS motifs binding 6–8 Ca²⁺ ions) and front-to-front N-terminal interactions stabilized by Glu55-Lys49 salt bridges, resulting in electronegative polymer networks capable of buffering up to 80 Ca²⁺ ions per molecule. These polymers compact further to accommodate higher Ca²⁺ loads and anchor to the ryanodine receptor (RyR1) via triadin and junctin, facilitating the close proximity needed for efficient Ca²⁺ release. CASQ1 polymerization allows massive storage of Ca²⁺ with low free [Ca²⁺] in the SR, protecting against osmotic stress, and serves as a dynamic sensor of luminal Ca²⁺: at high [Ca²⁺], polymer proximity inhibits RyR1 to preserve stores, while depolymerization upon release relieves inhibition to amplify excitation-contraction coupling. CASQ1 also modulates store-operated Ca²⁺ entry (SOCE) through reverse signaling with STIM1/2, coordinates reuptake by SERCA, and prevents Ca²⁺ depletion during muscle fatigue. Mutations in CASQ1 that impair its polymerization or anchoring disrupt SR Ca²⁺ handling, leading to pathological Ca²⁺ leaks, muscle weakness, and disorders such as malignant hyperthermia, environmental heat stroke, and tubular aggregate myopathy, which are characterized by fatal hypermetabolic crises. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.