- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
BAP1 Antibody [L1A7]
Cat.No.: F4801
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB, IHC, FCM |
| Reactivity |
|---|
| Rat, Mouse, Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
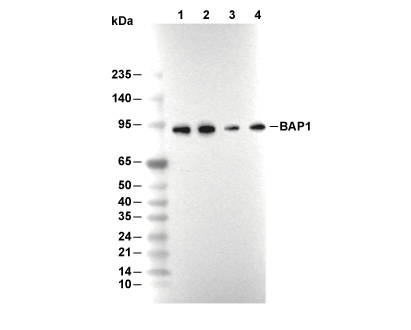
| Predicted MW Observed MW |
|---|
| 80 kDa 100 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | Human colon carcinoma; Human lung carcinoma tissue; Rat cerebrum tissue; Mouse cerebrum tissue; HeLa cells; PC-3 cells; A375 cells |
|---|---|
| Negative Control |
Biological Description
| Specificity |
|---|
| BAP1 Antibody [L1A7] detects endogenous levels of total BAP1 protein. |
| Clone |
|---|
| L1A7 |
| Synonym(s) |
|---|
| KIAA0272; hucep-6; BAP1; Ubiquitin carboxyl-terminal hydrolase BAP1; BRCA1-associated protein 1; Cerebral protein 6 |
| Background |
|---|
| BRCA1-associated protein 1 (BAP1) is a 729-amino-acid nuclear-cytoplasmic deubiquitinating enzyme (DUB) from the UCH family and acts as a potent tumor suppressor encoded by the BAP1 gene. It primarily localizes to the nucleus via C-terminal signals (amino acids 656–661 and 717–722) to regulate chromatin dynamics, DNA repair, and gene expression. Germline or somatic mutations in BAP1 underlie BAP1 tumor predisposition syndrome (BAP1-TPDS) and are implicated in cancers such as uveal melanoma, mesothelioma, renal cell carcinoma, and cutaneous melanomas. BAP1 contains an N-terminal catalytic UCH domain (amino acids 1–250; key residues Cys91, His169, Asp184 for deubiquitination), a ubiquitin-like (UBL) domain, and C-terminal binding motifs for ASXL1/2 (amino acids 635–693, forming the PR-DUB complex), HCF-1 (amino acids 365–385), BARD1 (amino acids 182–365), and BRCA1 (amino acids 596–721). These enable multiprotein interactions but BAP1 does not directly deubiquitinate BRCA1/BARD1; instead, it inhibits their E3 ligase activity through dimerization blockade. Functionally, BAP1 deubiquitinates H2AK119 (via the PR-DUB complex) to repress transcription (e.g., SLC7A11, promoting ferroptosis), stabilizes HCF-1 for cell cycle control, and acts on IP3R3 (in the cytoplasm) to promote Ca²⁺-dependent apoptosis. It also supports BRCA1/BARD1-independent DNA repair, the unfolded protein response under stress, metabolic regulation (NADPH balance, disulfidptosis suppression), and cellular differentiation. Biallelic inactivation of BAP1 in tumors disrupts these functions, resulting in genomic instability, immune evasion, and cancer progression. |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.