- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
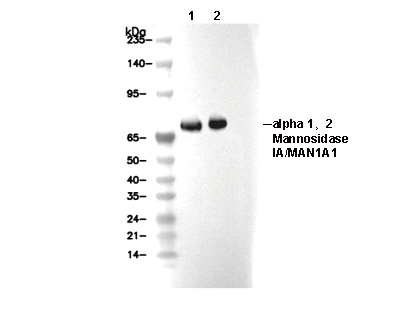
α 1,2 Mannosidase IA/MAN1A1 Antibody [H3N23]
Cat.No.: F3954
Application:
Reactivity:
Usage Information
| Dilution |
|---|
|
| Application |
|---|
| WB |
| Reactivity |
|---|
| Human |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Storage Buffer |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Storage (from the date of receipt) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Predicted MW Observed MW |
|---|
| 73 kDa 73 kDa |
| *Why do the predicted and actual molecular weights differ? The following reasons may explain differences between the predicted and actual protein molecular weight. |
| Positive Control | HepG2 cell; 293T cell |
|---|---|
| Negative Control |
Exprimental Methods
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/NP-40 Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:1000), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
Biological Description
| Specificity |
|---|
| α 1,2 Mannosidase IA/MAN1A1 Antibody [H3N23] detects endogenous levels of total α 1,2 Mannosidase IA/MAN1A1 protein. |
| Subcellular Location |
|---|
| Golgi apparatus, Membrane |
| Uniprot ID |
|---|
| P33908 |
| Clone |
|---|
| H3N23 |
| Synonym(s) |
|---|
| Man(9)-alpha-mannosidase; Mannosidase alpha class 1A member 1; Man9-mannosidase; MAN1A1 |
| Background |
|---|
| α 1,2 Mannosidase IA (MAN1A1) is a class I Golgi α1,2-mannosidase of glycosyl hydrolase family 47 (GH47) that acts as a type II transmembrane enzyme essential for N-linked glycan maturation during glycoprotein processing. MAN1A1 features a short N-terminal cytoplasmic tail, a single transmembrane helix, and a luminal C-terminal catalytic domain forming a novel (α/α)₇ barrel, housing the active site with key acidic residues (Asp, Glu) and a stabilizing calcium ion. MAN1A1 sequentially hydrolyzes three α1,2-linked mannose residues from high-mannose Man₉GlcNAc₂ oligosaccharides in the cis/medial Golgi, producing Man₅GlcNAc₂ structures that act as substrates for GlcNAc-transferases, ultimately enabling the formation of hybrid and complex N-glycans critical for correct protein folding, trafficking, and cell surface recognition. This mannose trimming step is integral to the calnexin/calreticulin quality control cycle and the N-glycan biosynthesis pathway, helping to distinguish properly folded proteins from misfolded ones destined for ER-associated degradation (ERAD). Reduced MAN1A1 expression alters glycan profiles, enhances cancer cell adhesion to endothelium, and promotes metastasis, while dysregulation is implicated in congenital disorders of glycosylation (CDG). |
| References |
|---|
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.