research use only
Pitavastatin (NK-104) Calcium HMG-CoA Reductase inhibitor
Cat.No.S1759
Chemical Structure
Molecular Weight: 880.98
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other HMG-CoA Reductase Inhibitors | Mevastatin SR-12813 Clinofibrate Cerivastatin sodium Dihydrolanosterol 7-ketocholesterol |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293 | Function assay | Drug uptake in HEK293 cells expressing OATP1B1 (unknown origin) assessed as OATP1B1-mediated drug transport, Km = 4.8 μM. | 22587986 | |||
| hepatocytes | Function assay | 0.1 to 10 uM | up to 90 mins | Drug metabolism in Sprague-Dawley rat hepatocytes assessed per 10'6 cells at 0.1 to 10 uM up to 90 mins by media-loss method, Km = 13 μM. | 22593038 | |
| Neuro2a | Function assay | 1 uM | Inhibition of delta 8-7 isomerase in mouse Neuro2a cells assessed as decrease in 7-DHC levels at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| Neuro2a | Function assay | 1 uM | Inhibition of DR24 in mouse Neuro2a cells assessed as decrease in 7-DHC levels at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| Neuro2a | Function assay | 1 uM | Inhibition of HMGCoA reductase in Dhcr7-deficient mouse Neuro2a cells assessed as decrease in 7-DHC levels at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| MCF-7 | Function assay | 10 uM | 24 hrs | Antagonist activity at Myc-tagged RXRalpha (unknown origin) expressed in human MCF-7 cells assessed as inhibition of 9-cis-RA induced receptor transactivation at 10 uM after 24 hrs by luciferase reporter gene assay | 28089347 | |
| MCF-7 | Function assay | 1 uM | 24 hrs | Antagonist activity at Myc-tagged RXRalpha (unknown origin) expressed in human MCF-7 cells assessed as inhibition of 9-cis-RA induced receptor transactivation at 1 uM after 24 hrs by luciferase reporter gene assay | 28089347 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(113.5 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 880.98 | Formula | C50H46CaF2N2O8 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 147526-32-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NK-104 calcium | Smiles | C1CC1C2=NC3=CC=CC=C3C(=C2C=CC(CC(CC(=O)[O-])O)O)C4=CC=C(C=C4)F.C1CC1C2=NC3=CC=CC=C3C(=C2C=CC(CC(CC(=O)[O-])O)O)C4=CC=C(C=C4)F.[Ca+2] | ||
Mechanism of Action
| Targets/IC50/Ki |
cholesterol esters
HMG-CoA reductase
|
|---|---|
| In vitro |
Pitavastatin significantly reduces both intracellular levels and synthesis of cholesterol esters. Pitavastatin is found to enhance LDL-receptor expression in vitro, as well as the amount of LDL binding to the LDL-receptor. Pitavastatin also exhibits more potent induction of LDL receptor mRNA expression compared with simvastatin and atorvastatin. Pitavastatin has many pleiotropic effects in vitro and in vivo, including deterring progression of atherosclerosis via inhibition of thromboxane synthesis, inhibition of migration/proliferation of vascular smooth muscle cells induced by angiotensin II, and stabilization of atherosclerotic plaque.
Pitavastatin is able to activate PPARα and induce HDL apoA-I through inducing inhibition of the Rho-signaling pathway.
Pitavastatin (1 μM) treatment for 48 h is able to enhances bone morphogenetic protein-2 BMP-2 (2.5-fold) and osteocalcin (10-fold) expression by inhibition of Rho-associated kinase in human osteoblasts. Pitavastatin inhibits growth and colony formation of liver cancer Huh-7 cells and SMMC7721 cells. It induces arrest of liver cancer cells at the G1 phase. Increased proportion of sub-G1 cells is observed after pitavastatin treatment. Pitavastatin promotes caspase-9 cleavage and caspase-3 cleavage in liver cancer cells. Pitavastatin could regulate NF-κB and anti-inflammation in hepatocellular carcinoma cells. Pitavastatin could induce autophagic cell death in glioma cells and promote sensitivity of cells to radiotherapy. It could inhibit cell proliferation and induce cell apoptosis in cholangiocarcinoma cells as well.
|
| In vivo |
Pitavastatin decreases the tumor growth and improved the survival of tumor-bearing mice. Pitavastatin exerts a protective effect on dilated cardiomyopathy possibly through down-regulating the circulating and local RAS, followed by inhibition of PKCb2 phosphorylation, and consequently promoting the phosphorylation of PLB as well as the activity and the expressions of SERCA2a and RyR2, whereby heart function is preserved in the development of DCM.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | Nrf2 / NQO1 / HO-1 Flt1 / Flk1 VEGF / p-Akt / AKT / Jagged-1 / c-Notch1 / Notch-1 / Hes-1 |
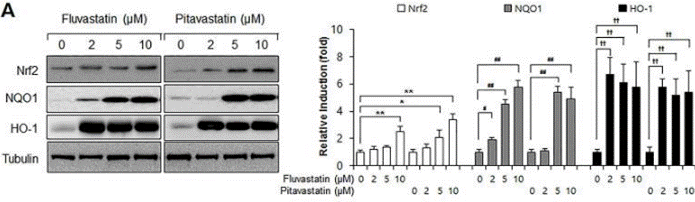
|
28542559 |
| Growth inhibition assay | Cell number |
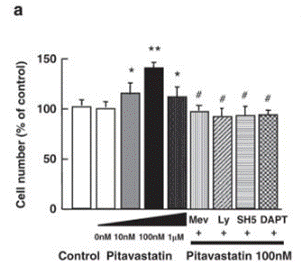
|
21301413 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05977738 | Recruiting | Glioblastoma Multiforme Adult|Recurrent Glioblastoma |
C.Dirven|Erasmus Medical Center |
January 18 2024 | Early Phase 1 |
| NCT04643093 | Completed | Primary Hypercholesterolemia|Mixed Dyslipidemias |
Orient Pharma Co. Ltd. |
August 1 2020 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
How to prepare the solution of it for in vivo use?
Answer:
You could use the formulation: 5% DMSO +40%PEG 300+5%Tween80+ddH2O for i.p., at a working concentration of 12.5mg/ml, stable for no longer than 40min.






































