research use only
TAK-243 (MLN7243) UAE inhibitor
Cat.No.S8341
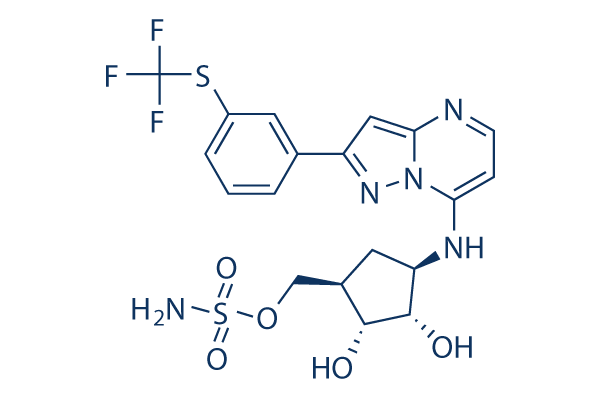
Chemical Structure
Molecular Weight: 519.52
Quality Control
| Related Targets | Proteasome E3 Ligase DUB p97 SUMO E2 conjugating |
|---|---|
| Other E1 Activating Inhibitors | MLN4924 (Pevonedistat) PYR-41 ML792 TAS4464 COH000 DKM 2-93 Pevonedistat hydrochloride PYZD-4409 NEDD8 inhibitor M22 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(192.48 mM)
Ethanol : 25 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 519.52 | Formula | C19H20F3N5O5S2 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1450833-55-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | N[S;v6](=O)(=O)OCC1CC(NC2=CC=NC3=CC(=N[N]23)C4=CC(=CC=C4)SC(F)(F)F)C(O)C1O | ||
Mechanism of Action
| Targets/IC50/Ki |
NF-κB
UAE
(Cell-free assay) 1 nM
|
|---|---|
| In vitro |
TAK-243 (MLN7243) treatment causes depletion of cellular ubiquitin conjugates, resulting in disruption of signaling events, induction of proteotoxic stress, and impairment of cell cycle progression and DNA damage repair pathways. This compound has weaker inhibitory activity against other closely related E1 ubiquitin-like activating enzymes such as Fat10-activating enzyme (UBA6; 7 ± 3 nM), NEDD8-activating enzyme (NAE; 28 ± 11 nM), SUMO-activating enzyme (SAE; 850 ± 180 nM), ISG15-activating enzyme (UBA7; 5,300 ± 2,100 nM) and autophagy-activating enzyme (ATG7; >10,000 nM) than it does against UAE. It inhibits UAE from transferring ubiquitin to an E2 enzyme. This chemical shows equally potent inhibition of the two E1 enzymes capable of activating ubiquitin (UBA6 and UAE), as indicated by comparable decreases in levels of charged USE1 and UBCH10. Downstream UAE pathway inhibition by this agent is evident, as shown by a dose- and time-dependent loss of both polyubiquitin chains and mono-ubiquitylated histone H2B; however, this treatment does not affect UAE (UBE1) protein levels. It also causes accumulation of short-lived proteins such as c-Jun, c-Myc, MCL1 and p53. |
| In vivo |
TAK-243 (MLN7243) treatment causes death of cancer cells and, in primary human xenograft studies. This compound demonstrates broad antitumor activity in models of solid and hematological tumors. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | poly-ubiquitin / ubiquitin / cleaved PARP PERK / GRP78 / p-eIF2α / CHOP / p-IκBα / PARP c-Myc / Mcl-1 |
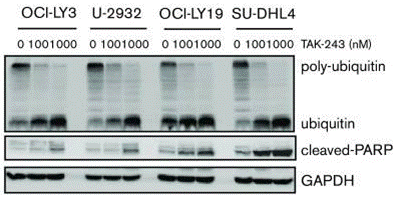
|
30617217 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03816319 | Not yet recruiting | Myelodysplastic Syndrome|Recurrent Acute Myeloid Leukemia|Refractory Acute Myeloid Leukemia|Refractory Chronic Myelomonocytic Leukemia|Refractory High Risk Myelodysplastic Syndrome |
National Cancer Institute (NCI) |
August 30 2024 | Phase 1 |
| NCT02045095 | Terminated | Advanced Malignant Solid Tumors |
Millennium Pharmaceuticals Inc.|Takeda |
January 31 2014 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































