research use only
Dabigatran (BIBR-1048) etexilate Thrombin inhibitor
Cat.No.S2154
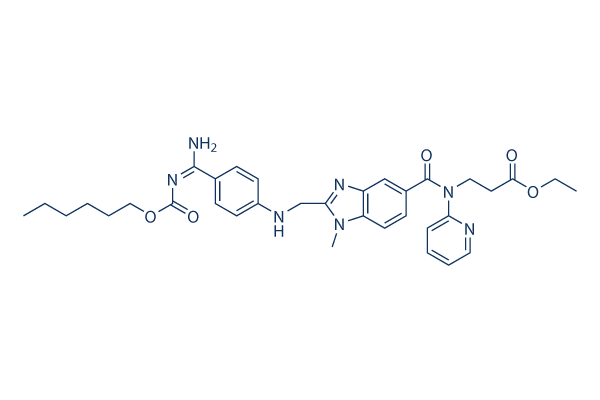
Chemical Structure
Molecular Weight: 627.73
Quality Control
| Related Targets | HDAC Caspase Proteasome Secretase MMP HCV Protease Cysteine Protease Tyrosinase DPP HIV Protease |
|---|---|
| Featured Inhibitors | Y-27632 Dihydrochloride SB431542 CHIR-99021 (Laduviglusib) RMC-7977 RMC-6236 (Daraxonrasib) MRTX1133 MG132 Z-VAD-FMK VT3989 IAG933 |
Solubility
|
In vitro |
DMSO
: 126 mg/mL
(200.72 mM)
Ethanol : 12 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 627.73 | Formula | C34H41N7O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 211915-06-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BIBR-1048 | Smiles | CCCCCCOC(=O)N=C(C1=CC=C(C=C1)NCC2=NC3=C(N2C)C=CC(=C3)C(=O)N(CCC(=O)OCC)C4=CC=CC=N4)N | ||
Mechanism of Action
| Targets/IC50/Ki |
Thrombin
|
|---|---|
| In vitro |
Dabigatran selectively and reversibly inhibits human thrombin (Ki: 4.5 nM) as well as thrombin-induced platelet aggregation (IC50: 10 nM), while showing no inhibitory effect on other platelet-stimulating agents. Dabigatran selectively and reversibly inhibits human thrombin (Ki: 4.5 nM) as well as thrombin-induced platelet aggregation (IC50: 10 nM), while showing no inhibitory effect on other platelet-stimulating agents. Dabigatran inhibits thrombin generation in platelet-poor plasma (PPP) with IC50 of 0.56 μM, measured as the endogenous thrombin potential (ETP). Dabigatran demonstrates concentrationdependent anticoagulant effects in various species in vitro, doubling the activated partial thromboplastin time (aPTT), prothrombin time (PT) and ecarin clotting time (ECT) in human PPP at concentrations of 0.23, 0.83 and 0.18 μM, respectively.
|
| In vivo |
Dabigatran prolongs the aPTT dose-dependently after intravenous administration in rats (0.3, 1 and 3 mg/kg) and rhesus monkeys (0.15, 0.3 and 0.6 mg/kg). Dabigatran etexilate (20 mg/kg, orally) produces less prolongation of K value and less decreases in angle and maximum amplitude than enoxaparin in swine. Dabigatran (0.01-0.1 mg/kg) reduces thrombus formation dose-dependently, with an ED50 (50% of the effective dose) of 0.033 mg/kg and complete inhibition at 0.1 mg/kg. Dabigatran etexilate (5-30 mg/kg) inhibits thrombus formation in a dose- and time-dependent manner, with maximum inhibition within 30 min of pretreatment, suggesting a rapid onset of action.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06360055 | Recruiting | Health |
Peking University Third Hospital |
April 1 2024 | Not Applicable |
| NCT05966740 | Recruiting | Venous Thromboembolism |
Boehringer Ingelheim|Children''s Hospital Acquired Thrombosis consortium |
October 31 2023 | -- |
| NCT05673889 | Completed | Healthy |
Pfizer|Arvinas Estrogen Receptor Inc. |
January 27 2023 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































