research use only
Ulixertinib (BVD-523) ERK1/2 inhibitor
Cat.No.S7854
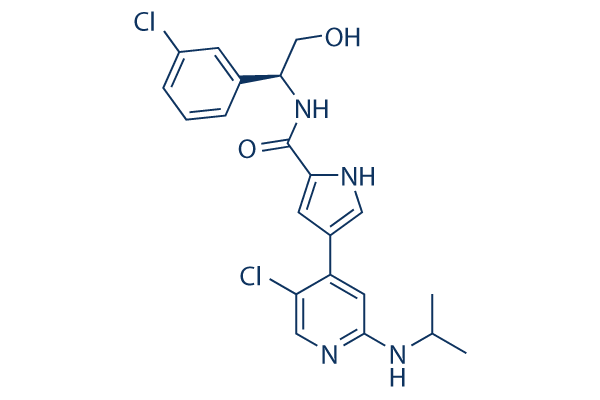
Chemical Structure
Molecular Weight: 433.33
Quality Control
Products Often Used Together with Ulixertinib (BVD-523)
| Related Targets | p38 MAPK Raf JNK MEK Ras KRas S6 Kinase MAP4K TAK1 Mixed Lineage Kinase |
|---|---|
| Other ERK Inhibitors | Ravoxertinib (GDC-0994) SCH772984 LY3214996 (Temuterkib) FR 180204 VX-11e XMD8-92 AZD0364 (ATG-017) ERK5-IN-1 Senkyunolide I MK-8353 (SCH900353) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| A375 | Function assay | Inhibition of ERK2 in human A375 cells harboring BRAF V600E mutant assessed as decrease in phosphorylated RSK levels, IC50 = 0.031 μM. | 28376306 | |||
| A375 | Function assay | 2 hrs | Inhibition of ERK1/2 in human A375 cells harboring B-RAF V600E mutant assessed as decrease in phospho-RSK level after 2 hrs by Cellomics ArrayScanTM VTI imaging analysis, IC50 = 0.14 μM. | 25977981 | ||
| A375 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A375 cells harboring B-RAF V600E mutant after 72 hrs by Cellomics ArrayScanTM VTI imaging analysis, IC50 = 0.18 μM. | 25977981 | ||
| SKCO1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKCO1 cells harboring KRAS G12V mutant after 72 hrs by CellTiter-Glo assay, IC50 = 0.356 μM. | 28038940 | ||
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells harboring KRAS G12D mutant after 72 hrs by CellTiter-Glo assay, IC50 = 0.468 μM. | 28038940 | ||
| SW620 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW620 cells harboring KRAS G12V mutant after 72 hrs by CellTiter-Glo assay, IC50 = 0.499 μM. | 28038940 | ||
| AsPC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human AsPC1 cells harboring KRAS G12D mutant after 72 hrs by CellTiter-Glo assay, IC50 = 0.849 μM. | 28038940 | ||
| NCI-H23 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H23 cells harboring KRAS G12C mutant after 72 hrs by CellTiter-Glo assay, IC50 = 1 μM. | 28038940 | ||
| SW156 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SW156 cells harboring wild type KRAS after 72 hrs by CellTiter-Glo assay, IC50 = 1.24 μM. | 28038940 | ||
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells after 72 hrs by CellTiter-Glo assay, IC50 = 2.231 μM. | 28038940 | ||
| A375 | Function assay | 2 hrs | Inhibition of ERK2 in human A375 cells harboring B-RAF V600E mutant assessed as decrease in phospho-ERK2 level after 2 hrs by Cellomics ArrayScanTM VTI imaging analysis, IC50 = 4.1 μM. | 25977981 | ||
| A375 | Function assay | Inhibition of ERK2 in human A375 cells harboring BRAF V600E mutant assessed as decrease in phosphorylated ERK2 levels, IC50 = 4.1 μM. | 28376306 | |||
| BA/F3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against mouse BA/F3 cells harboring KRAS G12D mutant after 72 hrs in presence of IL-3 by CellTiter-Glo assay, IC50 = 8.608 μM. | 28038940 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 86 mg/mL
(198.46 mM)
Ethanol : 86 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 433.33 | Formula | C21H22Cl2N4O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 869886-67-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | VRT752271 | Smiles | CC(C)NC1=NC=C(C(=C1)C2=CNC(=C2)C(=O)NC(CO)C3=CC(=CC=C3)Cl)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
ERK1
ERK2
<0.3 nM
|
|---|---|
| In vitro |
In an A375 melanoma cell line containing a b-RAFV600E mutation, Ulixertinib (BVD-523) reduces the levels of phosphorylated ERK2 (pERK) and of the phosphorylation of the downstream kinase RSK (pRSK) with IC50 of 4.1/0.14 μM, respectively. It also inhibits A375 cell proliferation with IC50 of 180 nM.
|
| Kinase Assay |
ERK2 Rapidfire Mass Spectrometry Inhibition of Catalysis Assay
|
|
MEK U911-activated ERK2 protein is expressed and purified in-house. Enzyme and substrate solutions are made up in assay buffer consisting of 50 mM Tris (pH 7.5), 10 mM MgCl2, 0.1 mM EGTA, 10 mM DTT and 0.01% (v/v) CHAPS. 1.2 nM ERK2 protein is prepared in assay buffer and 10 µL is dispensed into each well of a polypropylene, 384-well plate containing test and reference control compounds, including Ulixertinib (BVD-523). The compound plates had previously been dosed with a 12 point range from 100 µM down to 0.1 nM in order to calculate its IC50s, with a total DMSO concentration in the assay of 1%. Following a 20 minute pre-incubation of enzyme and compound at room temperature, 10 µL of substrate solution is added consisting of 16 µM Erktide (IPTTPITTTYFFFK) and 120 µM ATP (measured Km) in assay buffer. The reaction is allowed to progress for 20 minutes at room temperature before being quenched by the addition of 80 µl 1% (v/v) formic acid. The assay plates are then run on the RapidFire Mass Spectrometry platform to measure substrate (unphosphorylated Erktide) and product (phosphorylated Erktide) levels.
|
References |
Applications
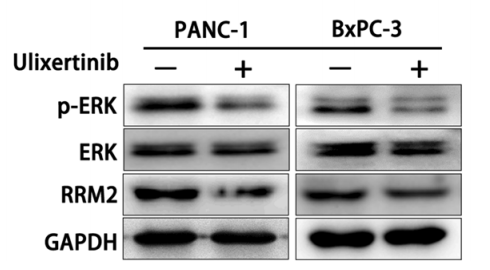
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-ERK / ERK / RRM2 |
|
28797284 |
| Growth inhibition assay | Cell viability |
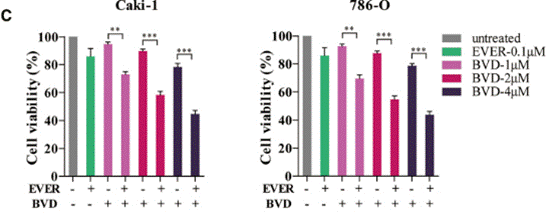
|
30771617 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04488003 | Terminated | Advanced Solid Tumor|BRAF Gene Mutation|BRAF Gene Alteration|MEK Mutation|MEK Alteration|MAP2K1 Gene Mutation|MAP2K1 Gene Alteration|MAP2K2 Gene Mutation|MAP2K2 Gene Alteration |
BioMed Valley Discoveries Inc |
November 3 2020 | Phase 2 |
| NCT04145297 | Completed | Gastrointestinal Neoplasms |
University of Utah|BioMed Valley Discoveries Inc |
March 17 2020 | Phase 1 |
| NCT03698994 | Active not recruiting | Advanced Malignant Solid Neoplasm|Recurrent Ependymal Tumor|Recurrent Ewing Sarcoma|Recurrent Glioma|Recurrent Hepatoblastoma|Recurrent Histiocytic and Dendritic Cell Neoplasm|Recurrent Langerhans Cell Histiocytosis|Recurrent Malignant Germ Cell Tumor|Recurrent Malignant Solid Neoplasm|Recurrent Medulloblastoma|Recurrent Neuroblastoma|Recurrent Non-Hodgkin Lymphoma|Recurrent Osteosarcoma|Recurrent Peripheral Primitive Neuroectodermal Tumor|Recurrent Primary Malignant Central Nervous System Neoplasm|Recurrent Rhabdoid Tumor|Recurrent Rhabdomyosarcoma|Recurrent Soft Tissue Sarcoma|Refractory Ependymoma|Refractory Ewing Sarcoma|Refractory Glioma|Refractory Hepatoblastoma|Refractory Histiocytic and Dendritic Cell Neoplasm|Refractory Langerhans Cell Histiocytosis|Refractory Malignant Germ Cell Tumor|Refractory Malignant Solid Neoplasm|Refractory Medulloblastoma|Refractory Neuroblastoma|Refractory Non-Hodgkin Lymphoma|Refractory Osteosarcoma|Refractory Peripheral Primitive Neuroectodermal Tumor|Refractory Primary Malignant Central Nervous System Neoplasm|Refractory Rhabdoid Tumor|Refractory Rhabdomyosarcoma|Refractory Soft Tissue Sarcoma|Wilms Tumor |
National Cancer Institute (NCI) |
November 14 2018 | Phase 2 |
| NCT03417739 | Active not recruiting | Uveal Melanoma |
Dana-Farber Cancer Institute|BioMed Valley Discoveries Inc |
March 26 2018 | Phase 2 |
| NCT02994732 | Completed | Healthy |
BioMed Valley Discoveries Inc |
January 2017 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































