research use only
S-Ruxolitinib (INCB018424) JAK inhibitor
Cat.No.S2902
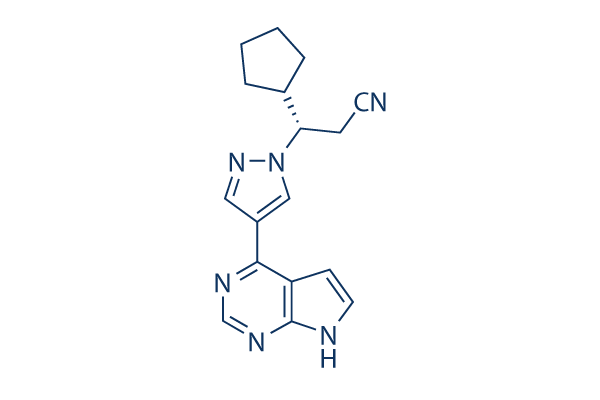
Chemical Structure
Molecular Weight: 306.37
Quality Control
| Related Targets | EGFR STAT Pim |
|---|---|
| Other JAK Inhibitors | BMS-986165 (Deucravacitinib) AZD1480 WP1066 Filgotinib (GLPG0634) Momelotinib (CYT387) AT9283 Gandotinib (LY2784544) Pacritinib TG101209 Cerdulatinib (PRT062070) hydrochloride |
Solubility
|
In vitro |
DMSO
: 61 mg/mL
(199.1 mM)
Ethanol : 61 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 306.37 | Formula | C17H18N6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 941685-37-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3 | ||
Mechanism of Action
| Targets/IC50/Ki |
JAK2
(Cell-free assay) 2.8 nM
JAK1
(Cell-free assay) 3.3 nM
TYK2
(Cell-free assay) 19 nM
|
|---|---|
| In vitro |
S-Ruxolitinib (INCB018424) potently and selectively inhibits JAK2V617F-mediated signaling and proliferation in Ba/F3 cells and HEL cells. It markedly increases apoptosis in a dose dependent manner in Ba/F3 cells. This compound (64 nM) results in a doubling of cells with depolarized mitochondria in Ba/F3 cells. It inhibits proliferating of erythroid progenitors from normal donors and polycythemia vera patients with IC50 of 407 nM and 223 nM, respectively. INCB018424 demonstrates remarkable potency against erythroid colony formation with IC50 of 67nM.
|
| Kinase Assay |
Binding assay
|
|
Recombinant proteins are expressed using Sf21 cells and baculovirus vectors and purified with affinity chromatography. JAK kinase assays use a homogeneous time-resolved fluorescence assay with the peptide substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with S-Ruxolitinib (INCB018424) or control, JAK enzyme, 500 nM peptide, adenosine triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour. The 50% inhibitory concentration (IC50) is calculated as the concentration of this compound required for inhibition of 50% of the fluorescent signal.
|
|
| In vivo |
S2902 (180 mg/kg, orally, twice a day) results in survive rate of greater than 90% by day 22 in a JAK2V617F-driven mouse model. S2902(180 mg/kg, orally, twice a day) markedly reduces splenomegaly and circulating levels of inflammatory cytokines, and preferentially eliminated neoplastic cells, resulting in significantly prolonged survival without myelosuppressive or immunosuppressive effects in a JAK2V617F-driven mouse model. The primary end point is reached in 41.9% of patients in the S2902 group as compared with 0.7% in the placebo group in the double-blind trial of myelofibrosis. Ruxolitinib results in maintaining of reduction in spleen volume and improvement of 50% or more in the total symptom score. A total of 28% of the patients in the S2902 (15 mg twice daily) group has at least a 35% reduction in spleen volume at week 48 in patients with myelofibrosis, as compared with 0% in the group receiving the best available therapy. The mean palpable spleen length has decreased by 56% with S2902 but has increased by 4% with the best available therapy at week 48. Patients in the S2902 group has an improvement in overall quality-of-life measures and a reduction in symptoms associated with myelofibrosis.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06310304 | Active not recruiting | Healthy Participants |
Incyte Corporation |
March 26 2024 | Phase 1 |
| NCT02596347 | Completed | Chronic Beryllium Disease (CBD)|Beryllium Sensitization (BeS) |
National Jewish Health |
April 2015 | -- |
| NCT00617994 | Completed | Psoriasis |
Incyte Corporation |
August 31 2007 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































