research use only
Filgotinib (GLPG0634) JAK inhibitor
Cat.No.S7605
Chemical Structure
Molecular Weight: 425.50
Quality Control
| Related Targets | EGFR STAT Pim |
|---|---|
| Other JAK Inhibitors | BMS-986165 (Deucravacitinib) AZD1480 WP1066 Momelotinib (CYT387) AT9283 Gandotinib (LY2784544) Pacritinib (SB1518) TG101209 Cerdulatinib (PRT062070) hydrochloride NVP-BSK805 2HCl |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human CD34+ cells | Function assay | 45 mins | Inhibition of JAK2 homodimer in human CD34+ cells spiked into human whole blood assessed as inhibition of EPO-induced STAT-5 phosphorylation preincubated for 45 mins followed by EPO addition measured after 15 mins by FACS analysis | 24417533 | ||
| NK92 | Function assay | Inhibition of JAK1/JAK3 in IL2-induced human NK92 cells assessed as pSTAT5, =0.148μM. | 25369270 | |||
| THP1 | Function assay | Inhibition of JAK1/JAK3 in IL4-induced human THP1 cells assessed as pSTAT6, =0.154μM. | 25369270 | |||
| U2OS | Function assay | Inhibition of JAK1/TYK2 in IFN-alphaB2-induced human U2OS cells assessed as pSTAT1, =0.436μM. | 25369270 | |||
| HeLa | Function assay | Inhibition of JAK1/JAK2 in OSM-induced human HeLa cells assessed as STAT1 reporter, =1.045μM. | 25369270 | |||
| THP1 | Function assay | Inhibition of JAK1/JAK2 in IFNgamma-induced human THP1 cells assessed as pSTAT1, =3.364μM. | 25369270 | |||
| TF1 | Function assay | Inhibition of JAK2 in IL3-induced human TF1 cells assessed as pSTAT5, =3.524μM. | 25369270 | |||
| BaF3 | Function assay | Inhibition of JAK2 in IL3-induced human BaF3 cells assessed as cell proliferation, =4.546μM. | 25369270 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 21 mg/mL
(49.35 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 425.50 | Formula | C21H23N5O3S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1206161-97-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CC1C(=O)NC2=NN3C(=N2)C=CC=C3C4=CC=C(C=C4)CN5CCS(=O)(=O)CC5 | ||
Mechanism of Action
| Targets/IC50/Ki |
JAK1
(Cell-free assay) 10 nM
JAK2
(Cell-free assay) 28 nM
TYK2
(Cell-free assay) 116 nM
JAK3
(Cell-free assay) 810 nM
|
|---|---|
| In vitro |
Filgotinib (GLPG0634) inhibits IL-2- and IL-4-induced JAK1/JAK3/γc signaling and IFN-αB2-induced JAK1/TYK2 type II receptor signaling with IC50 values ranging from 150 to 760 nM in cell lines. It shows higher selectivity for JAK/STAT signaling involving JAK1 than JAK2 kinase in a cellular context. Besides, this compound also inhibits the differentiation of Th1, Th2, and Th17 cells.
|
| In vivo |
Following oral administration, the absolute bioavailability of filgotinib (GLPG0634) is moderate in rats (45%) and high in mice (∼100%). This compound (30 mg/kg daily (Rats); 50 mg/kg twice daily (Mice)) dose-dependently reduces inflammation, cartilage, and bone degradation in the CIA model in rats and mice. In DSS-treated mice, it demonstrates that inhibition of JAK1 is sufficient for achieving strong efficacy in pre-clinical mouse model, correlated to the inhibition of STAT3 phosphorylation in the inflamed colon.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-STAT3 / STAT3 |
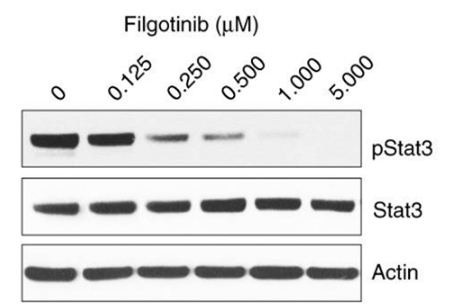
|
28191885 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06222034 | Recruiting | Juvenile Idiopathic Arthritis |
Galapagos NV |
April 2024 | Phase 1 |
| NCT06043739 | Completed | Bioavailability |
Galapagos NV |
September 22 2023 | Phase 1 |
| NCT05697159 | Recruiting | Rheumatoid Arthritis|Sickness Behavior|Inflammatory Disease|Autoimmune|Pain Chronic |
NHS Greater Glasgow and Clyde|Galapagos NV |
August 22 2023 | -- |
| NCT05653791 | Active not recruiting | Ulcerative Colitis |
Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Galapagos NV |
October 1 2022 | -- |
| NCT03417778 | Completed | Rheumatoid Arthritis |
Gilead Sciences|Galapagos NV |
April 3 2018 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Could you recommend a vehicle for oral gavage for it?
Answer:
It can be dissolved in 5% Tween 80+0.5% CMC Na at 30 mg/ml as a suspension for oral gavage.






































