|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C13H12FN3O3S |
||||||||||
| Molecular Weight | 309.32 | CAS No. | 178606-66-1 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 31 mg/mL (100.21 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | U-104 (MST-104, NSC 213841, SLC-0111, WBI-5111) is a potent carbonic anhydrase (CA) inhibitor for CA IX and CA XII with Ki of 45.1 nM and 4.5 nM, respectively, very low inhibition for CA I and CA II. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | U-104 (50 μM) blocks the mesenchymal phenotype in the cancer stem cells population in hypoxia condition of 4T1 cells. This compound (<50 μM) significantly reduces migration in a dose-dependent manner in metastatic MDA-MB-231 LM2-4Luc+ cells , with cells growing as compact colonies similar to parental MDA-MB-231 cells. This chemical possesses aromatic groups at the second nitrogen ureido group. | ||||
| In vivo | U-104 (38 mg/kg) inhibits primary tumor growth in the mice implanted orthotopically with MDA-MB-231 LM2-4Luc+ cells. This compound (19 mg/kg) inhibits metastases formation in the 4T1 experimental metastasis mice model. This chemical (38 mg/kg) significantly delay primary tumor growth and reduces cancer stem cell population in NOD/SCID mice orthotopically implanted with MDA-MB-231 LM2-4Luc+ cells. It (5 mg/mL, oral gavage) shows a significant delay in tumor growth in Balb/c mice orthotopically implanted with 4T1 cells. |
Protocol (from reference)
| Animal Study:[2] |
|
|---|
References
|
Customer Product Validation
-
, , Lab Invest, 2016, 96(12):1234-1245
-
Data from [ , , Sci Rep, 2015, 5:14508 ]
-
Data from [ , , Int J Oncol, 2018, 53(1):189-202 ]
Selleck's U-104 (SLC-0111) Has Been Cited by 11 Publications
| Alterations in gene expression associated with invasion of RAS-mutant thyroid tumors and their potential diagnostic and therapeutic utility [ Eur Thyroid J, 2025, 14(3)e250022] | PubMed: 40440326 |
| Depletion of slow-cycling PDGFRα+ADAM12+ mesenchymal cells promotes antitumor immunity by restricting macrophage efferocytosis [ Nat Immunol, 2023, 24(11):1867-1878] | PubMed: 37798557 |
| SLC-0111, an inhibitor of carbonic anhydrase IX, attenuates hepatoblastoma cell viability and migration [ Front Oncol, 2023, 13:1118268] | PubMed: 36776327 |
| CA9 and PRELID2; hypoxia-responsive potential therapeutic targets for pancreatic ductal adenocarcinoma as per bioinformatics analyses [ J Pharmacol Sci, 2023, 10.1016/j.jphs.2023.10.003] | PubMed: 37973221 |
| A nanoplatform to boost multi-phases of cancer-immunity-cycle for enhancing immunotherapy [ J Control Release, 2021, 339:403-415] | PubMed: 34655676 |
| Hotspots of Aberrant Enhancer Activity in Fibrolamellar Carcinoma Reveal Candidate Oncogenic Pathways and Therapeutic Vulnerabilities. [ Cell Rep, 2020, 14;31(2):107509] | PubMed: 32294439 |
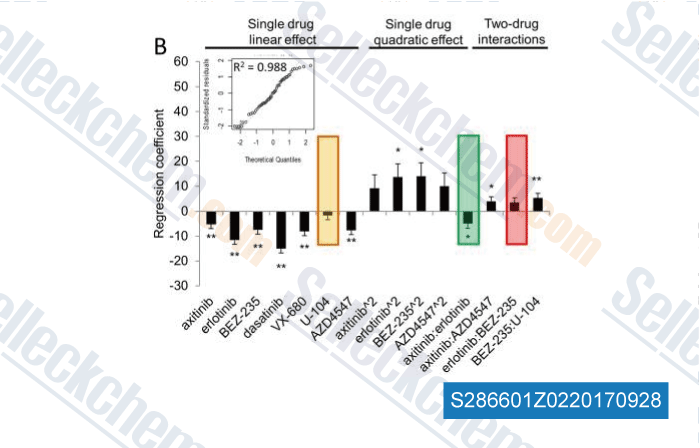
| Integrating Phenotypic Search and Phosphoproteomic Profiling of Active Kinases for Optimization of Drug Mixtures for RCC Treatment [ Cancers (Basel), 2020, 12(9)E2697] | PubMed: 32967224 |
| One-pot synthesis of pH-responsive charge-switchable PEGylated nanoscale coordination polymers for improved cancer therapy [Yang Y, et al. Biomaterials, 2018, 156:121-133] | PubMed: 29195181 |
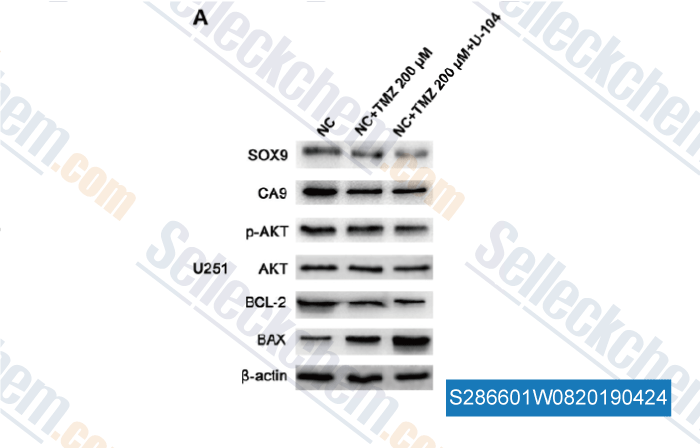
| Association between SOX9 and CA9 in glioma, and its effects on chemosensitivity to TMZ [Xu X, et al. Int J Oncol, 2018, 53(1):189-202] | PubMed: 29749469 |
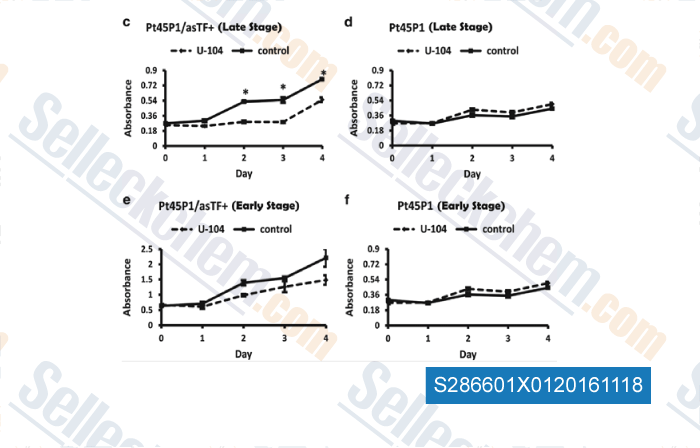
| Activation of carbonic anhydrase IX by alternatively spliced tissue factor under late-stage tumor conditions [Ramchandani D, et al. Lab Invest, 2016, 10.1038/labinvest.2016.103] | PubMed: 27721473 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
