|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C24H25FO5S |
||||||
| Molecular Weight | 444.52 | CAS No. | 842133-18-0 | ||||
| Solubility (25°C)* | In vitro | DMSO | 89 mg/mL (200.21 mM) | ||||
| Ethanol | 89 mg/mL (200.21 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Canagliflozin (TA 7284, JNJ 28431754) is a highly potent and selective SGLT2 inhibitor for hSGLT2 with IC50 of 2.2 nM in a cell-free assay, exhibits 413-fold selectivity over hSGLT1. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | Canagliflozin (JNJ 28431754) is a novel C-glucoside with thiophene ring. It inhibits Na+-dependent 14C-AMG uptake in a concentration-dependent fashion. This compound inhibits 14C-AMG uptake in CHO-hSGLT1 and mSGLT1 cells with IC50 of 0.7 μM and >1 μM, respectively. It inhibits the facilitative (non-Na+-linked) GLUT-mediated 2H-2-DG uptake in L6 myoblasts by less than 50%. In sham-injected oocytes, Canagliflozin (10 μM) or phlorizin (3 mM) alone in the presence of 50 μM DNJ does not affect currents. In SGLT3-injected oocytes, DMSO and Canagliflozin 10 μM inhibits DNJ-induced currents by 15.6% and 23.4%, respectively. | ||||||
| In vivo | Canagliflozin (JNJ 28431754) shows pronounced anti-hyperglycemic effects in high-fat diet fed KK (HF-KK) mice. Oral administration at 30 mg/kg of this compound to male SD rats induces glucose excretion over 24 hours by 3,696 mg per 200 g body weight. Pharmacokinetic studies reveals a much higher exposure following oral administration. Following intravenous and oral doses of 3 and 10 mg/kg, respectively, to male SD rats, AUC0−inf, po, t1/2 and oral bioavailability are determined to be 35,980 ng·h/mL, 5.2 hours, and 85%, respectively. Thus, inhibition of SGLT2 in renal tubules after oral dosing is likely to continuously suppress reabsorption of glucose. The extensive UGE would reflect excellent pharmacokinetic properties in vivo as well as high potency of SGLT2 inhibition. Since most of the filtered glucose is reabsorbed by SGLT2 in the renal tubules, the novel compound would be useful for an anti-diabetic agent. Single oral administration at 3 mg/kg remarkably reduced blood glucose levels without influencing food intake in hyperglycemic high-fat diet fed KK (HF-KK) mice. There is a 48% reduction in blood glucose level versus vehicle at 6 hours. In contrast, it only slightly affects blood glucose levels in normoglycemic mice. Therefore, Canagliflozin would control hyperglycemia in the therapy of T2DM with low risk of hypoglycemia. |
Protocol (from reference)
| Cell Assay:[1] |
|
|---|---|
| Animal Study:[2] |
|
References
|
Customer Product Validation

-
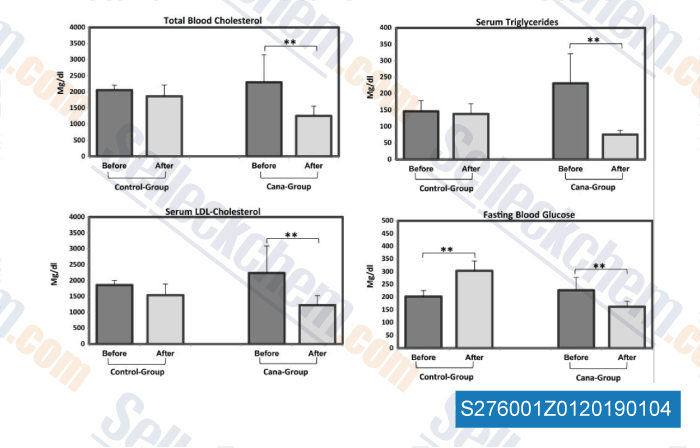
Data from [ , , Mol Metab, 2016, 5(10):1048-56 ]
-
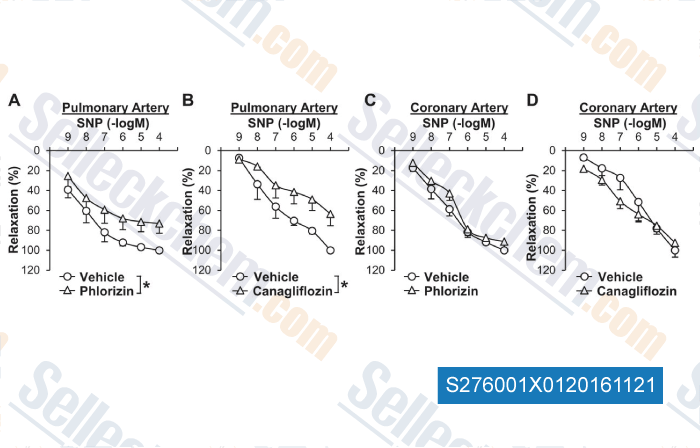
Data from [ , , Cardiovasc Diabetol, 2018, 17(1):106 ]
-
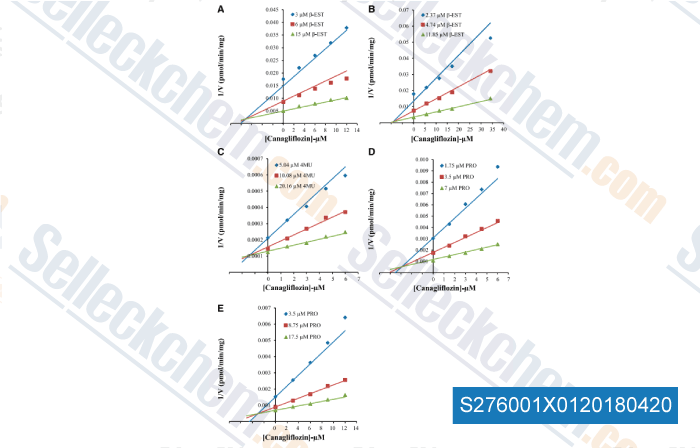
Data from [ , , Am J Physiol Lung Cell Mol Physiol, 2015, 309(9):L1027-36. ]
-
Data from [ , , Drug Metab Dispos, 2015, 43(10):1468-76. ]
Selleck's Canagliflozin (JNJ-28431754) Has Been Cited by 34 Publications
| Nuclear translocation of metabolic enzyme PKM2 participates in high glucose-promoted HCC metastasis by strengthening immunosuppressive environment [ Redox Biol, 2024, 71:103103] | PubMed: 38471282 |
| Canagliflozin reverses doxorubicin-induced cardiotoxicity via restoration of autophagic homeostasis [ Toxicol Appl Pharmacol, 2024, 495:117183] | PubMed: 39631538 |
| Canagliflozin primes antitumor immunity by triggering PD-L1 degradation in endocytic recycling [ J Clin Invest, 2023, 133(1)e154754] | PubMed: 36594471 |
| Canagliflozin ameliorates hypobaric hypoxia-induced pulmonary arterial hypertension by inhibiting pulmonary arterial smooth muscle cell proliferation [ Clin Exp Hypertens, 2023, 10.1080/10641963.2023.2278205] | PubMed: 37970663 |
| The impact of SGLT2 inhibitors on αKlotho in renal MDCK and HK-2 cells [ Front Endocrinol (Lausanne), 2023, 14:1069715] | PubMed: 36967770 |
| Study on the pharmacological mechanisms of sodium-glucose co-transporter 2 inhibitors in obesity-related atrial fibrillation based on network pharmacology and experimental verification [ Ann Transl Med, 2023, 11(8):300] | PubMed: 37181345 |
| Systematic identification of biomarker-driven drug combinations to overcome resistance [ Nat Chem Biol, 2022, 10.1038/s41589-022-00996-7] | PubMed: 35332332 |
| SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma [ Cell Death Dis, 2022, 13(6):523] | PubMed: 35662245 |
| Multi-site desmoplastic small round cell tumors are genetically related and immune-cold [ Cell Mol Life Sci, 2022, 79(5):273] | PubMed: 35503137 |
| Anti-inflammatory effect of SGLT-2 inhibitors viauric acidand insulin [ Cellular and Molecular Life Sciences, 2022, 273] | PubMed: None |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
