|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C20H21FN6O5 |
||||||
| Molecular Weight | 444.42 | CAS No. | 518048-05-0 | ||||
| Solubility (25°C)* | In vitro | DMSO | 89 mg/mL (200.26 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Raltegravir is a potent integrase (IN) inhibitor for WT and S217Q PFV IN with IC50 of 90 nM and 40 nM in cell-free assays, respectively. It shows greater than 1000-fold selectivity for HIV-1 IN over several related Mg2+-dependent enzyme such as HCV polymerase, HIV reverse transcriptase, HIV RNaseH and human α-, β-, γ-polymerases. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | PFV IN carrying the S217H substitution is 10-fold less susceptible to Raltegravir with IC50 of 900 nM. PFV IN displays 10% of WT activity and is inhibited by this compound with an IC50 of 200 nM, indicating a ~twofold decrease in susceptibility to the IN strand transfer inhibitor (INSTI) compared with WT IN. S217Q PFV IN is as sensitive to this compound as the WT enzyme. It is metabolized by glucuronidation, not hepatically. This compound has potent in vitro activity against HIV-1, with a 95% inhibitory concentration of 31?0 nM, in human T lymphoid cell cultures. It is also active against HIV-2 when tested in CEMx174 cells, with an IC95 of 6 nM. Its metabolism occurs primarily through glucuronidation. Drugs that are strong inducers of the glucuronidation enzyme, UGT1A1, significantly reduce its concentrations and should not be used. It exhibits weak inhibitory effects on hepatic cytochrome P450 activity. It does not induce CYP3A4 RNA expression or CYP3A4-dependent testosterone 6-β-hydroxylase activity. Its cellular permeativity is reduced in the presence of magnesium and calcium. This compound and related HIV-1 integrase (IN) strand transfer inhibitors (INSTIs efficiently block viral replication. In acutely infected human lymphoid CD4+ T-cell lines MT-4 and CEMx174, SIVmac251 replication is efficiently inhibited by this compound, which shows an EC90 in the low nanomolar range. | ||||
| In vivo | Raltegravir induces viro-immunological improvement of nonhuman primates with progressing SIVmac251 infection. One non-human primate shows an undetectable viral load following this compound monotherapy. | ||||
| Features | The 1st approved human immunodeficiency virus type 1 (HIV-1) integrase inhibitor. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
-
Data from [ Vet Microbiol , 2011 , 152(1-2), 165-8 ]
-
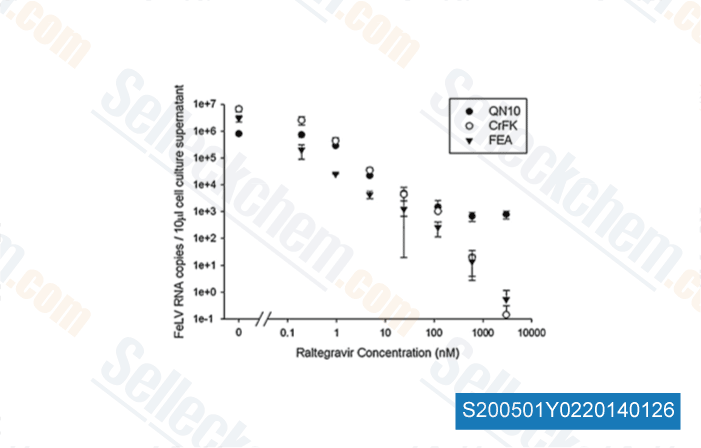
Data from [ , , Antimicrob Agents Chemother, 2014, 58(8): 4431-42 ]
Selleck's Raltegravir Has Been Cited by 76 Publications
| The macrophage-intrinsic MDA5/IRF5 axis drives HIV-1 intron-containing RNA-induced inflammatory responses [ J Clin Invest, 2025, 135(16)e187663] | PubMed: 40493408 |
| Pomalidomide enhances CD8+ T and NK cell mediated killing of HIV-infected cells [ EBioMedicine, 2025, 122:106004] | PubMed: 41232235 |
| Combined dendritic cell and anti-TIGIT immunotherapy potentiates adaptive NK cells against HIV-1 [ EMBO Mol Med, 2025, 10.1038/s44321-025-00255-x] | PubMed: 40473838 |
| Targeting Ikaros and Aiolos with pomalidomide fails to reactivate or induce apoptosis of the latent HIV reservoir [ J Virol, 2025, e0167624.] | PubMed: 39902962 |
| Limitations and use of the Morpheus-V5 dual reporter virus in assessing interventions that target HIV latency [ J Virol Methods, 2025, 338:115236] | PubMed: 40754007 |
| 5' cap sequestration is required for sensing of unspliced HIV-1 RNA by MDA5 [ bioRxiv, 2025, 2025.08.20.671346] | PubMed: 40909469 |
| IKAROS expression drives the aberrant metabolic phenotype of macrophages in chronic HIV infection [ Clin Immunol, 2024, 260:109915] | PubMed: 38286172 |
| Broad synergistic antiviral efficacy between a novel elite controller-derived dipeptide and antiretrovirals against drug-resistant HIV-1 [ Front Cell Infect Microbiol, 2024, 14:1334126] | PubMed: 38915925 |
| HMGB1 Expression Levels Correlate with Response to Immunotherapy in Non-Small Cell Lung Cancer [ Lung Cancer, 2024, 15:55-67] | PubMed: 38741920 |
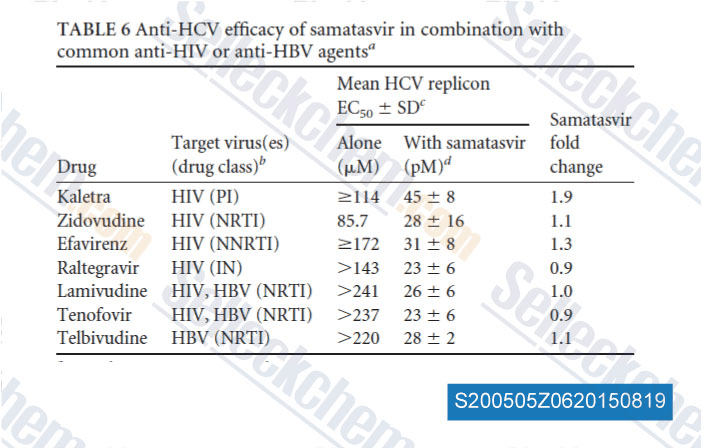
| Conversion of raltegravir carrying a 1,3,4-oxadiazole ring to a hydrolysis product upon pH changes decreases its antiviral activity [ PNAS Nexus, 2024, 3(1):pgad446] | PubMed: 38170115 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
