|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C14H19BCl2N2O4 |
||||||
| Molecular Weight | 361.03 | CAS No. | 1072833-77-2 | ||||
| Solubility (25°C)* | In vitro | DMSO | 72 mg/mL (199.42 mM) | ||||
| Ethanol | 72 mg/mL (199.42 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Ixazomib (MLN2238) inhibits the chymotrypsin-like proteolytic (β5) site of the 20S proteasome with IC50 and Ki of 3.4 nM and 0.93 nM in cell-free assays, respectively, also inhibits the caspase-like (β1) and trypsin-like (β2) proteolytic sites, with IC50 of 31 and 3500 nM. Ixazomib (MLN2238) induces autophagy. Phase 3. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | At higher concentrations, this compound also inhibits the caspase-like (β1) and trypsin-like (β2) proteolytic sites with IC50 of 31nM and 3.5uM, respectively. It inhibits Calu-6 cell with IC50 of 9.7 nM. MLN2238 is a selective, potent, and reversible inhibitor of the proteasome in tumor cells. This compound shows time-dependent reversible proteasome inhibition. Both this compound and Bortezomib shows time-dependent reversible proteasome inhibition; however, the proteasome dissociation half-life for it is determined to be ∼6-fold faster than that of Bortezomib (18 and 110 minutes, respectively). It dissociates more rapidly from the proteasome than Bortezomib, consistent with faster recovery of proteasome activity observed in the Proteasome-Glo assay. It has a greater overall tumor pharmacodynamic effect than Bortezomib as assessed by 20S inhibition. This compound is the biologically active form of MLN9708. | ||||
| In vivo | MLN2238 induces a greater pharmacodynamic response than Bortezomib in xenograft tumors. This compound shows greater maximum and sustained tumor proteasome inhibition compared with Bortezomib in xenograft models. These results confirm that the improved tumor exposure seen with this agent translates into an improved tumor pharmacodynamic response both at the level of and downstream from the proteasome. It shows antitumor activity in the CWR22 xenograft model. This chemical shows greater tumor pharmacodynamic responses in WSU-DLCL2 xenografts compared with Bortezomib. Similarly, Bortezomib treatment only led to a minor increase in GADD34 levels in WSU-DLCL2 xenograft tumors, whereas it strongly induces its expression. This compound has an improved pharmacodynamic profile and antitumor activity compared with Bortezomib in both OCI-Ly10 and PHTX22L models. | ||||
| Features | A first-in-class proteasome inhibitor that has improved pharmacokinetics (PK), pharmacodynamics(PD), and antitumor activity in preclinical studies. |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[2] |
|
References
|
Customer Product Validation
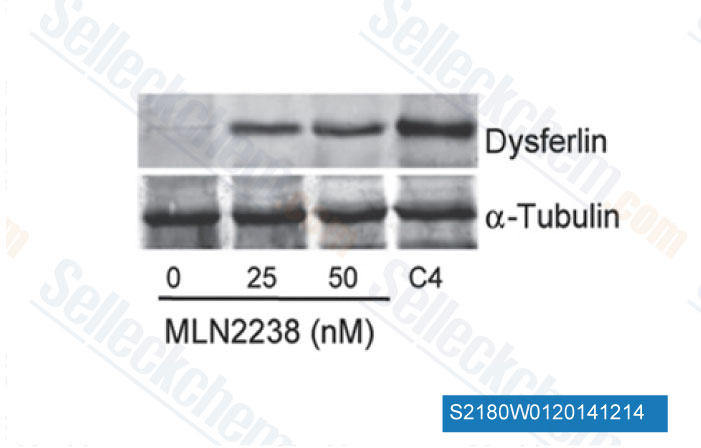
-
Data from [ Sci Transl Med , 2014 , 6(250), 250ra112 ]
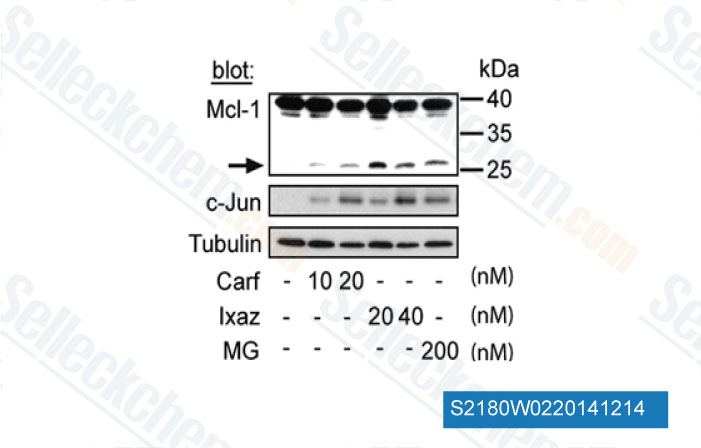
-
Data from [ Cancer Lett , 2014 , 343(2), 286-94 ]
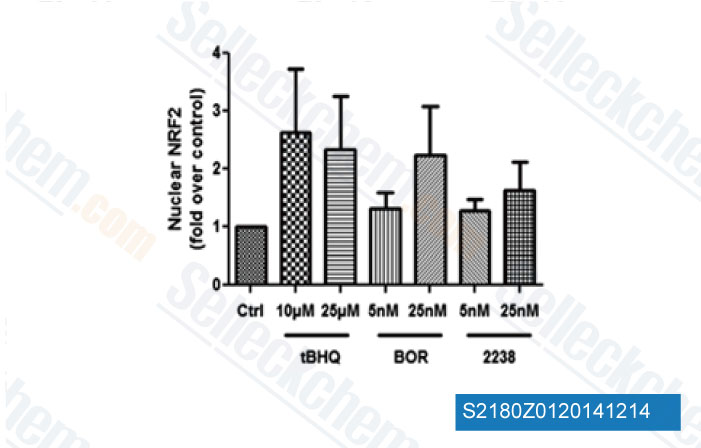
-
Data from [ Hemoglobin , 2014 , 38(3), 188-95 ]
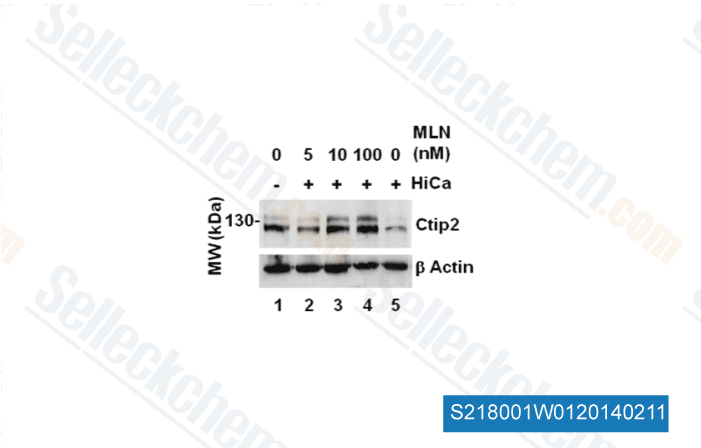
-
Data from [ J Cell Sci , 2012 , 125(Pt 23), 5733-44 ]
Selleck's MLN2238 (Ixazomib) Has Been Cited by 71 Publications
| Structural basis for allosteric modulation of M. tuberculosis proteasome core particle [ Nat Commun, 2025, 16(1):3138] | PubMed: 40169579 |
| A patient-derived T cell lymphoma biorepository uncovers pathogenetic mechanisms and host-related therapeutic vulnerabilities [ Cell Rep Med, 2025, S2666-3791(25)00102-8] | PubMed: 40147445 |
| Enhancing T cell cytotoxicity in multiple myeloma with bispecific αPD-L1 × αCD3 T cell engager-armed T cells and low-dose bortezomib therapy [ Biomed Pharmacother, 2025, 184:117878] | PubMed: 39891948 |
| High-Throughput Drug Screening of Clear Cell Ovarian Cancer Organoids Reveals Vulnerability to Proteasome Inhibitors and Dinaciclib and Identifies AGR2 as a Therapeutic Target [ Cancer Res Commun, 2025, 5(6):1018-1033] | PubMed: 40459063 |
| Integrated transcriptomics- and structure-based drug repositioning identifies drugs with proteasome inhibitor properties [ Sci Rep, 2024, 14(1):18772] | PubMed: 39138277 |
| A combinatorial therapeutic approach to enhance FLT3-ITD AML treatment [ Cell Rep Med, 2023, 10.1016/j.xcrm.2023.101286] | PubMed: 37951217 |
| Targeting ITGB4/SOX2-driven lung cancer stem cells using proteasome inhibitors [ iScience, 2023, 26(8):107302] | PubMed: 37554452 |
| Dual inhibition of HSF1 and DYRK2 impedes cancer progression [ Biosci Rep, 2023, 43(1)BSR20222102] | PubMed: 36622366 |
| Proteasome inhibition as a therapeutic target for the fungal pathogen Cryptococcus neoformans [ Microbiol Spectr, 2023, 11(5):e0190423] | PubMed: 37750732 |
| Proteasome inhibition as a therapeutic target for the fungal pathogen Cryptococcus neoformans [ Microbiol Spectr, 2023, 10.1128/spectrum.01904-23] | PubMed: 37750732 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
