|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C17H15FN2O3 |
||||||||||||||
| Molecular Weight | 314.31 | CAS No. | 852475-26-4 | ||||||||||||
| Solubility (25°C)* | In vitro | DMSO | 22 mg/mL (69.99 mM) | ||||||||||||
| Water | Insoluble | ||||||||||||||
| Ethanol | Insoluble | ||||||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||||||
Preparing Stock Solutions
Biological Activity
| Description | MC1568 is a selective HDAC inhibitor for maize HD1-A with IC50 of 100 nM in a cell-free assay. It is 34-fold more selective for HD1-A than HD1-B. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | MC1568 is a selective class II (IIa) histone deacetylas (HDAC II) inhibitor with IC50 of 220 nM and 176-fold class II selectivity (against class I). In human breast cancer ZR-75.1 cell lysates, MC1568 (5 μM) shows no inhibitory activity against HDAC1 but is able to inhibit HDAC4. [1] In MCF-7 cells, MC1568 (20 μM) increases the accumulation of acetylated H3 and H4 histones, as well as the levels of acetyl-tubulin, which indicates a inhibitory effect of MC1568 on HDAC6. [2] In C2C12 cells, MC1568 (5 μM) arrests myogenesis by decreasing myocyte enhancer factor 2D (MEF2D) expression, stabilizing the HDAC4-HDAC3-MEF2D complex, and by inhibiting differentiation-induced MEF2D acetylation. [3] MC1568 (5 or 10 μM) interferes with the RAR- and PPARγ-mediated differentiation-inducing signaling pathways. In F9 cells, MC1568 specifically blocks endodermal differentiation despite not affecting retinoic acid-induced maturation of promyelocytic NB4 cells. In 3T3-L1 cells, MC1568 attenuates PPARγ-induced adipogenesis. [4] |
||||
| In vivo | In mice, MC1568 (50 mg/kg) shows an apparent tissue-selective HDAC inhibition. In skeletal muscle and heart, MC1568 inhibits the activity of HDAC4 and HDAC5 without affecting HDAC3 activity, thereby leaving MEF2-HDAC complexes in a repressed state. [3] In reporting PPRE-Luc mice, MC1568 (50 mg/kg) impairs PPARγ signaling mostly in the heart and adipose tissues. [4] In a recent study of pancreatic explants, MC1568 enhances expression of Pax4, a key factor required for proper β-and δ-cell differentiation and amplifies endocrine β- and δ-cells. [5] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation

-
Data from [ Proc Natl Acad Sci U S A , 2012 , 109(34) ]
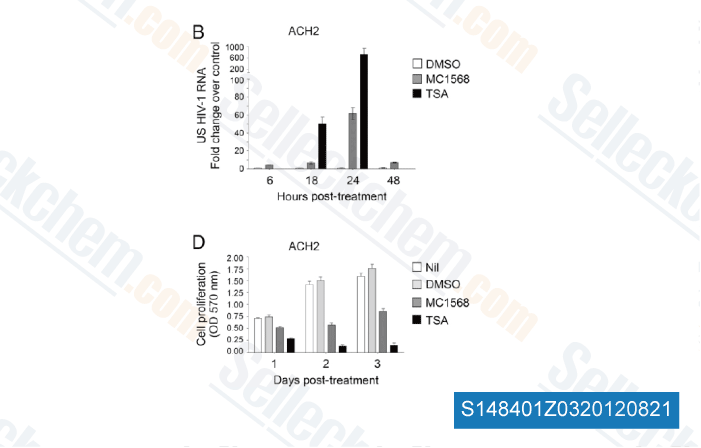
-
Data from [ Proc Natl Acad Sci U S A , 2012 , 109(34) ]
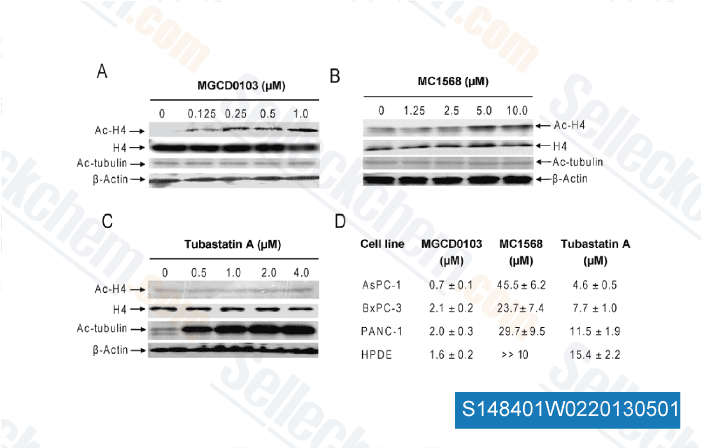
-
Data from [ PLoS One , 2012 , 7(12), e52095 ]
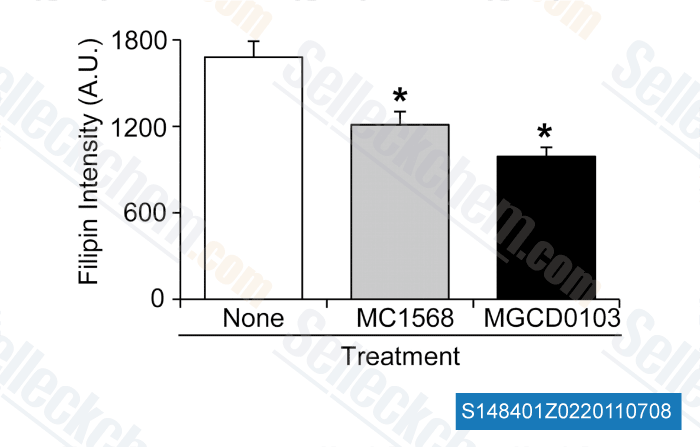
-
Data from [ J Biol Chem , 2011 , 286, 23842–23851 ]
Selleck's MC1568 Has Been Cited by 52 Publications
| HSD17B4 deficiency causes dysregulation of primary cilia and is alleviated by acetyl-CoA [ Nat Commun, 2025, 16(1):2663] | PubMed: 40102401 |
| Key epigenetic and signaling factors in the formation and maintenance of the blood-brain barrier [ Elife, 2024, 12RP86978] | PubMed: 39670988 |
| Global isonicotinylome analysis identified SMAD3 isonicotinylation promotes liver cancer cell epithelial-mesenchymal transition and invasion [ iScience, 2024, 27(9):110775] | PubMed: 39286495 |
| Selective Inhibition of Histone Deacetylase Class IIa With MC1568 Ameliorates Podocyte Injury [ Front Med (Lausanne), 2022, 9:848938] | PubMed: 35492337 |
| HBV covalently closed circular DNA minichromosomes in distinct epigenetic transcriptional states differ in their vulnerability to damage [ Hepatology, 2021, 10.1002/hep.32245] | PubMed: 34779008 |
| HBV covalently closed circular DNA minichromosomes in distinct epigenetic transcriptional states differ in their vulnerability to damage [ Hepatology, 2021, 10.1002/hep.32245] | PubMed: 34779008 |
| Butyrate and Class I Histone Deacetylase Inhibitors Promote Differentiation of Neonatal Porcine Islet Cells into Beta Cells [ Cells, 2021, 10(11)3249] | PubMed: 34831471 |
| Epigenetic Repression of Chloride Channel Accessory 2 Transcription in Cardiac Fibroblast: Implication in Cardiac Fibrosis [ Front Cell Dev Biol, 2021, 9:771466] | PubMed: 34869368 |
| HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. [ Nucleic Acids Res, 2020, 6;48(6):2912-2923] | PubMed: 31970414 |
| Valproate reverses mania-like behaviors in mice via preferential targeting of HDAC2 [ Mol Psychiatry, 2020, 10.1038/s41380-020-00958-2] | PubMed: 33235333 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
