|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C20H19F2N3O5 |
|||
| Molecular Weight | 419.38 | CAS No. | 1051375-16-6 | |
| Solubility (25°C)* | In vitro | DMSO | 84 mg/mL (200.29 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Dolutegravir is a two-metal-binding HIV integrase inhibitor with IC50 of 2.7 nM in a cell-free assay, modest activity against raltegravir-resistant signature mutants Y143R, Q148K, N155H, and G140S/Q148H. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | S/GSK1349572 shows the potent inhibitory effect on nine clinical isolates from integrase inhibitor-naive HIV-2-infected patients with EC50 ranging from 0.2 nM -1.4 nM. [1] In vitro, S/GSK1349572 inhibits recombinant HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM. Furthermore, S/GSK1349572 potently inhibits HIV replication in cells such as peripheral blood mononuclear cells (PBMCs), MT-4 cells and CIP4 cells infected with a self-inactivating PHIV lentiviral vector with EC50 of 0,51 nM, 0.71 nM and 2.2 nM, respectively. [2] In vitro, S/GSK1349572 exhibits potent activity against five different nonnucleoside reverse transcription inhibitor--resistant or nucleoside reverse transcription inhibitor--resistant viruses with EC50 ranging from 1.3 nM -2.1 nM. Similarly to that against wild-type virus, S/GSK1349572 shows equivalent activity against two protease inhibitor-resistant viruses with EC50 of 0.36 nM and 0.37 nM, respectively. [2] |
||
| In vivo | Dolutegravir (DTG) is a first-line antiretroviral drug (ARV) used in combination therapy for HIV-1. It inhibits MMP activity and has the potential to affect prenatal and postnatal neurodevelopment. |
||
| Features | A next-generation and two-metal-binding HIV integrase strand transfer inhibitor. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
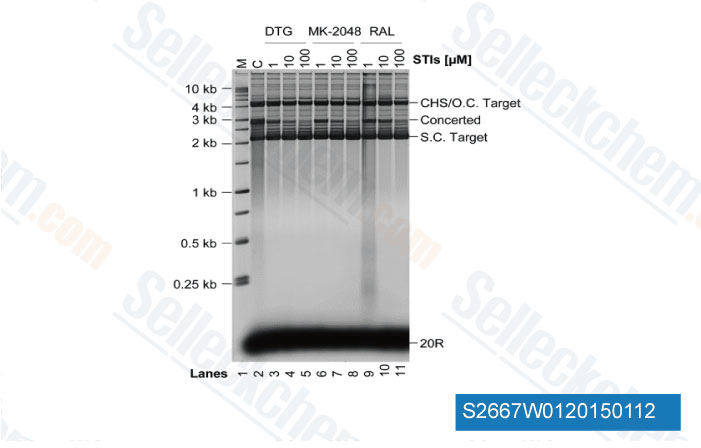
-
Data from [Data independently produced by J Biol Chem, 2014, 289(28), 19648-58]
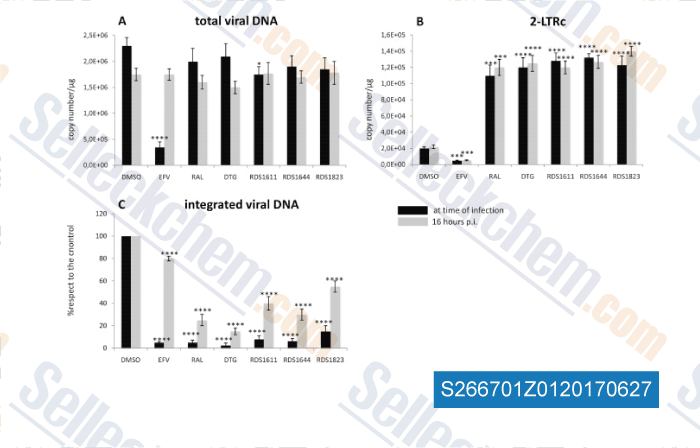
-
Data from [Data independently produced by , , Antiviral Res, 2016, 134:236-243]
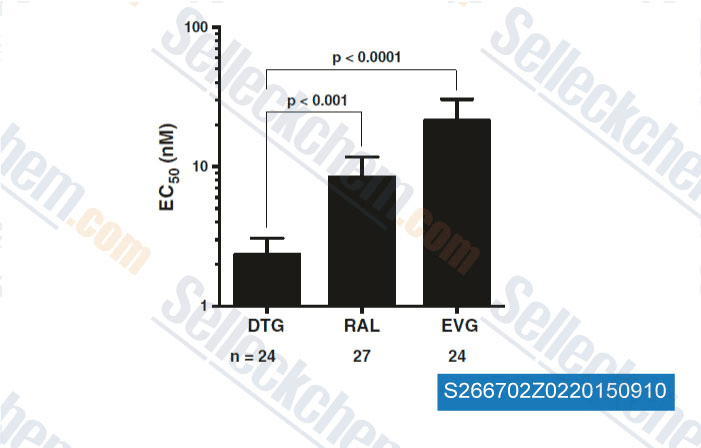
-
Data from [Data independently produced by , , Retrovirology, 2015, 10.1186/s12977-015-0146-8]
Selleck's Dolutegravir has been cited by 27 publications
| Biological and Structural Analyses of New Potent Allosteric Inhibitors of HIV-1 Integrase [ Antimicrob Agents Chemother, 2023, 67(7):e0046223] | PubMed: 37310224 |
| DNA ultra-sensitive quantification, a technology for studying HIV unintegrated linear DNA [ Cell Rep Methods, 2023, 3(4):100443] | PubMed: 37159665 |
| Spectrum of Activity of Raltegravir and Dolutegravir Against Novel Treatment-Associated Mutations In HIV-2 Integrase: A Phenotypic Analysis Using An Expanded Panel of Site-Directed Mutants [ J Infect Dis, 2022, jiac037] | PubMed: 35134180 |
| Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes [ Cells, 2022, 11(11)1841] | PubMed: 35681536 |
| In Vitro Susceptibility of HIV Isolates with High Growth Capability to Antiretroviral Drugs [ Int J Mol Sci, 2022, 23(23)15380] | PubMed: 36499705 |
| Reduction of CD8 T cell functionality but not inhibitory capacity by integrase inhibitors [ J Virol, 2022, JVI0173021] | PubMed: 35019724 |
| Effects of Injection Volume and Route of Administration on Dolutegravir In Situ Forming Implant Pharmacokinetics [ Pharmaceutics, 2022, 14(3)615] | PubMed: 35335991 |
| Impact of combinations of clinically observed HIV integrase mutations on phenotypic resistance to integrase strand transfer inhibitors (INSTIs): a molecular study [ J Antimicrob Chemother, 2022, dkab498] | PubMed: 35061879 |
| Biodegradable polymeric solid implants for ultra-long-acting delivery of single or multiple antiretroviral drugs [ Int J Pharm, 2021, 605:120844] | PubMed: 34216767 |
| A new engineering process of biodegradable polymeric solid implants for ultra-long-acting drug delivery [ Int J Pharm X, 2021, 3:100068] | PubMed: 33392498 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
