|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C25H32N8O |
||||||||||
| Molecular Weight | 460.57 | CAS No. | 802539-81-7 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 17 mg/mL (36.91 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Milciclib is a potent, ATP-competitive CDK inhibitor for CDK2 with IC50 of 45 nM. It is >3-fold more selective for CDK2 than CDK1, 2, 4, 5, and 7, it's also an inhibitor of TRKA with IC50 of 53nM. This compound induces cell death through autophagy. Phase 2. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
|||||||||||
| In vitro | PHA-848125 inhibits, although with lower potency, the activities of cyclin H/CDK7, cyclin D1/CDK4, p35/CDK5, as well as cyclin E/CDK2 and cyclin B/CDK1 with IC50 values of 0.15, 0.16, 0.265, 0.363, 0.398 μM, respectively. Thropomyosin receptor kinase A is also inhibited by this compound in the same nanomolar range as CDKs. In the most PHA-848125-sensitive cell line, this compound induces a concentration-dependent G(1) arrest. This compound also impairs phosphorylation of the retinoblastoma protein at CDK2 and CDK4 specific sites, reduces retinoblastoma protein and cyclin A levels, and increases p21(Cip1), p27(Kip1) and p53 expression. This chemical is added to the cells 48 h after TMZ and cell growth is evaluated after 3 additional days of culture. A drug combination of TMZ, BG and this compound induces an additive or synergistic effect on cell growth, depending on the cell line. In the absence of BG, the combination is still more active than the single agents in cell lines moderately sensitive to TMZ, but comparable to this compound alone in the two TMZ-resistant cell lines. When TMZ plus BG are used in combination with this chemical against cultured normal melanocytes, neither synergistic nor additive antiproliferative effects are observed. | |||||||||||
| In vivo | In the preclinical xenograft A2780 human ovarian carcinoma model, this compound reveals good efficacy and is well tolerated upon repeated daily treatments. Treatment of K-Ras(G12D)LA2 mice with this chemical (40 mg/kg twice daily for 10 days) results in significant tumor growth inhibition at the end of the treatment and is accompanied by a reduction in the cell membrane turnover. On the other hand, following oral administration, it shows significant antitumor activity in various human xenografts, carcinogen-induced tumors and in disseminated primary leukemia models; the plasma concentrations in rodents being in the same range as those found inhibiting cancer cell proliferation. |
Protocol (from reference)
| Kinase Assay:[4] |
|
|---|---|
| Cell Assay:[1] |
|
| Animal Study:[3] |
|
References
|
Customer Product Validation
-
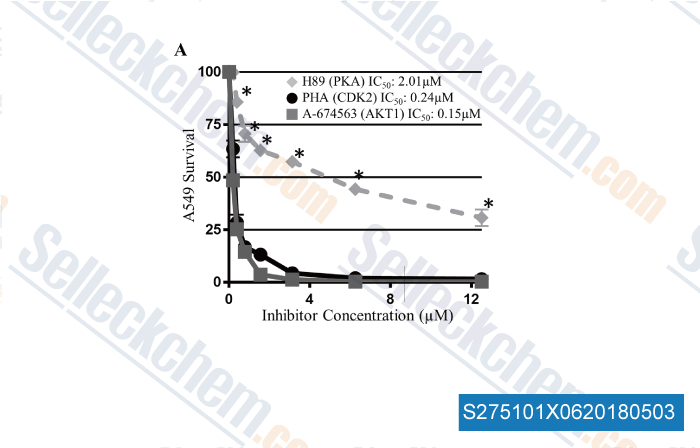
Data from [ , , PLoS ONE, 2018, 13(2): e0193344 ]
Selleck's Milciclib Has Been Cited by 19 Publications
| Combined therapy with DR5-targeting antibody-drug conjugate and CDK inhibitors as a strategy for advanced colorectal cancer [ Cell Rep Med, 2025, S2666-3791(25)00231-9] | PubMed: 40449480 |
| Spatial proteo-transcriptomic profiling reveals the molecular landscape of borderline ovarian tumors and their invasive progression [ medRxiv, 2023, 2023.11.13.23298409] | PubMed: 38014221 |
| Visual barcodes for clonal-multiplexing of live microscopy-based assays [ Nat Commun, 2022, 13(1):2725] | PubMed: 35585055 |
| Mechanisms Contributing to Animal Cell Size Homeostasis [ ProQuest, 2022, 29166130] | PubMed: none |
| The role of drug efflux and uptake transporters ABCB1 (P-gp), ABCG2 (BCRP) and OATP1A/1B and of CYP3A4 in the pharmacokinetics of the CDK inhibitor milciclib [ Eur J Pharm Sci, 2021, 159:105740] | PubMed: 33524505 |
| The role of drug efflux and uptake transporters ABCB1 (P-gp), ABCG2 (BCRP) and OATP1A/1B and of CYP3A4 in the pharmacokinetics of the CDK inhibitor milciclib [ Eur J Pharm Sci, 2021, S0928-0987(21)00042-7] | PubMed: 33524505 |
| Differential reprogramming of breast cancer subtypes in 3D cultures and implications for sensitivity to targeted therapy [ Sci Rep, 2021, 11(1):7259] | PubMed: 33790333 |
| Infant high grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. [ Cancer Discov, 2020, 1 pii: CD-19-1030] | PubMed: 32238360 |
| A Patient-Derived Cell Atlas Informs Precision Targeting of Glioblastoma [ Cell Rep, 2020, 32(2):107897] | PubMed: 32668248 |
| Tethering of Telomeres to the Nuclear Envelope Is Mediated by SUN1-MAJIN and Possibly Promoted by SPDYA-CDK2 During Meiosis [ Front Cell Dev Biol, 2020, 8:845] | PubMed: 33015044 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
