|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C15H13ClFNO2 |
|||
| Molecular Weight | 293.72 | CAS No. | 220991-20-8 | |
| Solubility (25°C)* | In vitro | DMSO | 59 mg/mL (200.87 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Lumiracoxib (COX-189) is a novel, selective COX-2 inhibitor with Ki of 0.06 μM. It also inhibits COX1 with Ki of 3 μM. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Lumiracoxib has an IC50 of 0.14 μm in COX-2-expressing dermal fibroblasts, but caused no inhibition of COX-1 at concentrations up to 30 μm (HEK 293 cells transfected with human COX-1). In a human whole blood assay, IC50 values for Lumiracoxib are 0.13 μM for COX-2 and 67 μM for COX-1 (COX-1/COX-2 selectivity ratio 515). [1] | ||||
| In vivo | Lumiracoxib is a highly selective COX-2 inhibitor with anti-inflammatory, analgesic and antipyretic activities comparable with diclofenac, the reference NSAID, but with much improved gastrointestinal safety. Lumiracoxib is rapidly absorbed following oral administration in rats with peak plasma levels being reached between 0.5 and 1 h. Efficacy of Lumiracoxib in rat models of hyperalgesia, oedema, pyresis and arthritis is dose-dependent and similar to diclofenac. However, consistent with its low COX-1 inhibitory activity, Lumiracoxib at a dose of 100 mg/kg orally causes no ulcers and is significantly less ulcerogenic than diclofenac. [1] |
Protocol (from reference)
| Animal Study:[1] |
|
|---|
Customer Product Validation
-
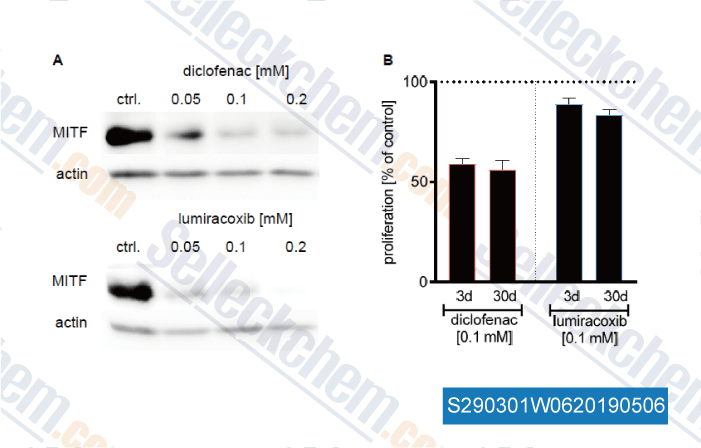
Data from [Data independently produced by , , Cancer Lett, 2019, 442:453-463]
-
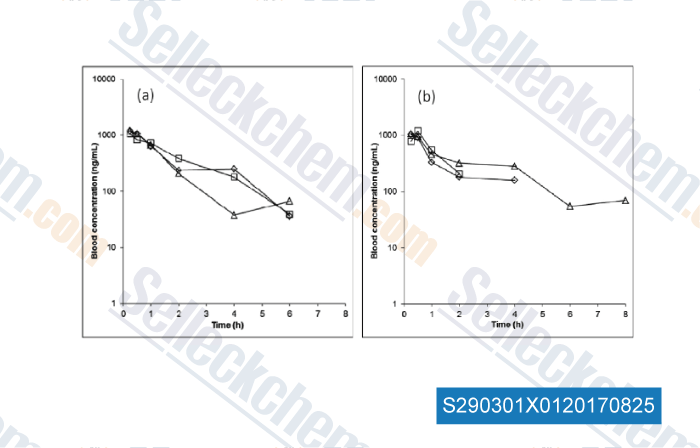
Data from [Data independently produced by , , Biochem Pharmacol, 2017, 135:139-150]
-
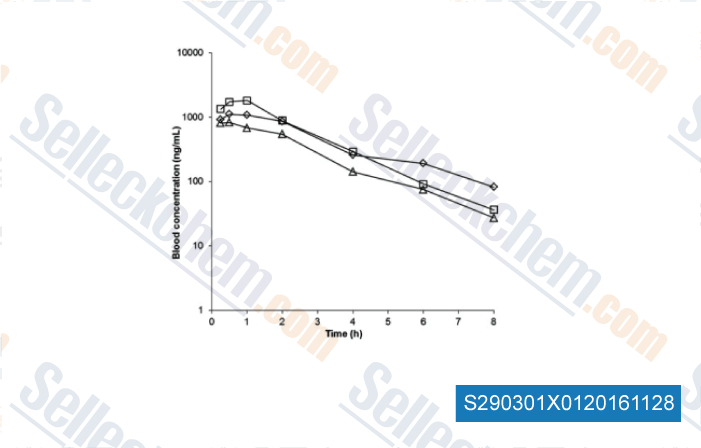
Data from [Data independently produced by , , Xenobiotica, 2016, 18:1-9.]
Selleck's Lumiracoxib has been cited by 12 publications
| Catalytically active phospholipase A2 myotoxin from Crotalus durissus terrificus induces proliferation and differentiation of myoblasts dependent on prostaglandins produced by both COX-1 and COX-2 pathways [ Int J Biol Macromol, 2021, 187:603-613] | PubMed: 34314795 |
| IL-1β and TNF-α Modulation of Proliferated and Committed Myoblasts: IL-6 and COX-2-Derived Prostaglandins as Key Actors in the Mechanisms Involved [ Cells, 2020, 9(9)E2005] | PubMed: 32882817 |
| Low-Dose Aspirin Treatment Attenuates Male Rat Salt-Sensitive Hypertension via Platelet Cyclooxygenase 1 and Complement Cascade Pathway. [ J Am Heart Assoc, 2020, 9(1):e013470] | PubMed: 31852420 |
| Metabolic targeting synergizes with MAPK inhibition and delays drug resistance in melanoma [Brummer C Cancer Lett, 2019, 442:453-463] | PubMed: 30481565 |
| Restricting Glycolysis Preserves T Cell Effector Functions and Augments Checkpoint Therapy. [ Cell Rep, 2019, 29(1):135-150] | PubMed: 31577944 |
| Cyclooxygenase-2 inhibition reduces anxiety-like behavior and normalizes enhanced amygdala glutamatergic transmission following chronic oral corticosterone treatment. [ Neurobiol Stress, 2019, 11:100190] | PubMed: 31467944 |
| Detection of Cyclooxygenase-2-Derived Oxygenation Products of the Endogenous Cannabinoid 2-Arachidonoylglycerol in Mouse Brain [ ACS Chem Neurosci, 2018, 9(7):1552-1559] | PubMed: 29722963 |
| The pharmacokinetics and metabolism of lumiracoxib in chimeric humanized and murinized FRG mice. [Dickie AP, et al. Biochem Pharmacol, 2017, 135:139-150] | PubMed: 28351678 |
| Lumiracoxib metabolism in male C57bl/6J mice: characterisation of novel in vivo metabolites [P Dickie A Xenobiotica, 2017, 47(6):538-546] | PubMed: 27430634 |
| Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. [ Elife, 2016, 5] | PubMed: 27162170 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
