|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C14H12N6O |
|||
| Molecular Weight | 280.28 | CAS No. | 141505-33-1 | |
| Solubility (25°C)* | In vitro | DMSO | 56 mg/mL (199.8 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Levosimendan(OR1259,OR1855,Simsndan) is a calcium sensitizer acting through calcium-dependent binding to cardiac troponin C (cTnC), provides treatment for heart failure. | |
|---|---|---|
| Targets |
|
|
| In vitro | Levosimendan is a calcium sensitizer acting through calcium-dependent binding to cardiac troponin C (cTnC). Levosimendan at 3 μM decreases the value of Ca50 from 2.73μM to 1.19 μM. levosimendan exhibits its calcium sensitizing effect through calcium-dependent binding to the N-terminal domain of cTnC. [1] Levosimendan significantly hyperpolarizes resting potential of rat mesenteric arterial myocytes with an EC50 of 2.9 μM and maximal effect (19.5 mV) at 10 μM, probably through activation of a glibenclamide-sensitive K+ channel. [2] Levosimendan has inotropic and lusitropic actions in failing human myocardium, with average maximum increase in twitch tension of 47% at a levosimendan concentration of 0.8 μM. [3] Levosimendan causes rapid dose-dependent improvement in hemodynamic function in patients with decompensated heart failure. [4] |
|
| In vivo | Levosimendan at low concentrations (0.03 to 0.1 μM) acts preferably as a Ca2+ sensitizer, whereas at higher concentrations (0.1 to 0.3 μmol/L) its action as a phosphodiesterase inhibitor contributes to the positive inotropic effect. [5] |
Protocol (from reference)
| Animal Study: |
|
|---|
References
Customer Product Validation
-
, , Antiviral Res, 2017, 146:76-85
Selleck's Levosimendan has been cited by 1 publication
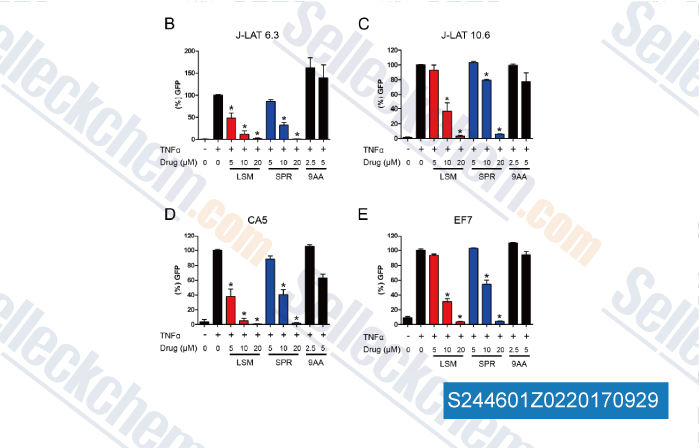
| Screening of an FDA-approved compound library identifies levosimendan as a novel anti-HIV-1 agent that inhibits viral transcription. [Hayashi T, et al. Antiviral Res, 2017, 146:76-85] | PubMed: 28842263 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
