|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C27H29NO10 . HCl |
|||
| Molecular Weight | 563.98 | CAS No. | 23541-50-6 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (177.31 mM) | |
| Water | 100 mg/mL (177.31 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Daunorubicin HCl inhibits both DNA and RNA synthesis and inhibits DNA synthesis with Ki of 0.02 μM in a cell-free assay. Daunorubicin is a topoisomerase II inhibitor that induces apoptosis. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | At drug concentrations that reflect the peak plasma concentration after Daunorubicin administration, the primary mechanism is likely to be through interaction with topoisomerase II, which may be a primary triggering event for growth arrest and/or cell killing through a signalling pathway leading to apoptosis, at least in leukemic cells and thymocytes. The quinone structure permits daunorubicin to act as electron acceptors in reactions mediated by oxoreductive enzymes including cytochrome P450 reductase, NADH dehydrogenase, and xanthine oxidase. At Daunorubicin concentrations exceeding approximately 2–4 μM, free radical mediated toxicity and DNA cross-linking may become evident. Daunorubicin inhibits both DNA and RNA syntheses in HeLa cells over a concentration range of 0.2 through 2 μM. Daunorubicin inhibits both DNA syntheses in Ehrlich ascites tumor cells over a concentration range of 4 μM. Daunorubic triggers apoptosis at concentrations of 0.5 and 1 μM in either HL-60 or U-937 human leukemic cells. [1] Daunorubicin stimulates ceramide elevation and apoptosis in P388 and U937 cells through de novo synthesis via activation of the enzyme ceramide synthase. [2] Daunorubicin dose-dependently increases the phosphatidylserine exposure and consequent procoagulant activity of human umbilical vein endothelial cells. Daunorubicin (0.2 mM) significantly enhances the release of endothelial microparticles which are highly procoagulant in human umbilical vein endothelial cells. [3] |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
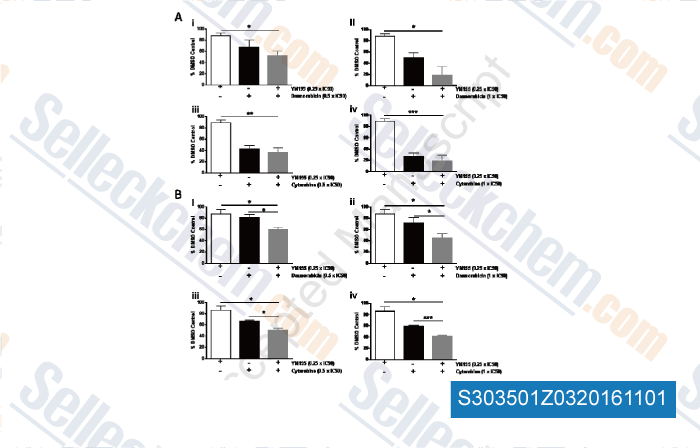
-
, , Leuk Res, 2015, 39(4):435-44.
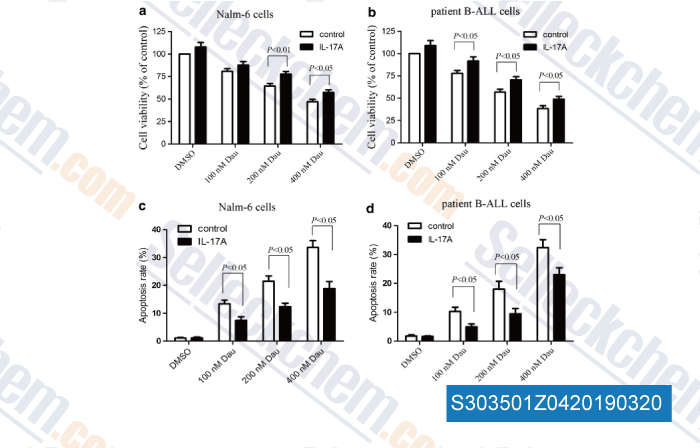
-
Data from [Data independently produced by , , J Transl Med, 2016, 14(1):132]
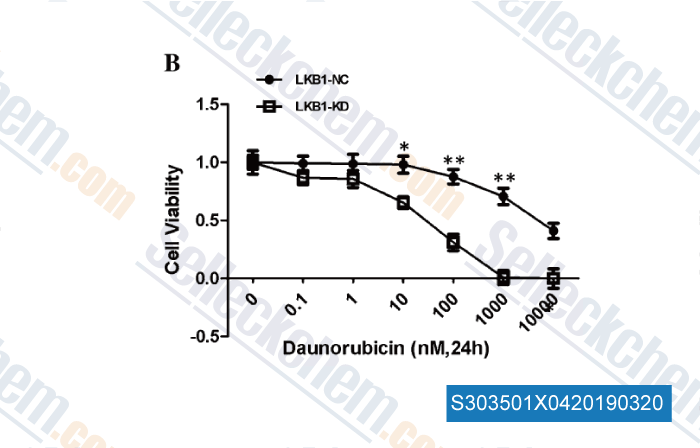
-
Data from [Data independently produced by , , Mol Cell Biochem, 2017, 434(1-2):25-32]
Selleck's Daunorubicin HCl has been cited by 68 publications
| SRCAP mutations drive clonal hematopoiesis through epigenetic and DNA repair dysregulation [ Cell Stem Cell, 2023, 10.1016/j.stem.2023.09.011] | PubMed: 37863054 |
| Transcriptional Response to Standard AML Drugs Identifies Synergistic Combinations [ Int J Mol Sci, 2023, 24(16)12926] | PubMed: 37629110 |
| Bone Marrow Microenvironment-Induced Chemoprotection in KMT2A Rearranged Pediatric AML Is Overcome by Azacitidine-Panobinostat Combination [ Cancers (Basel), 2023, 15(12)3112] | PubMed: 37370721 |
| Lower cardiotoxicity of CPX-351 relative to daunorubicin plus cytarabine free-drug combination in hiPSC-derived cardiomyocytes in vitro [ Sci Rep, 2023, 13(1):21054] | PubMed: 38030645 |
| P2RY2-AKT activation is a therapeutically actionable consequence of XPO1 inhibition in acute myeloid leukemia [ Nat Cancer, 2022, 3(7):837-851] | PubMed: 35668193 |
| Elucidating minimal residual disease of paediatric B-cell acute lymphoblastic leukaemia by single-cell analysis [ Nat Cell Biol, 2022, 10.1038/s41556-021-00814-7] | PubMed: 35145224 |
| Acquired mutations in BAX confer resistance to BH3-mimetic therapy in Acute Myeloid Leukemia [ Blood, 2022, blood.2022016090] | PubMed: 36219880 |
| Generating experimentally unrelated target molecule-binding highly functionalized nucleic-acid polymers using machine learning [ Nat Commun, 2022, 13(1):4541] | PubMed: 35927274 |
| Degradation of GSPT1 causes TP53-independent cell death in leukemia while sparing normal hematopoietic stem cells [ J Clin Invest, 2022, 132(16)e153514] | PubMed: 35763353 |
| Population-based high-throughput toxicity screen of human iPSC-derived cardiomyocytes and neurons [ Cell Rep, 2022, 39(1):110643] | PubMed: 35385754 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
