|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C28 H33 N7 O2 |
||||||||||
| Molecular Weight | 499.61 | CAS No. | 1421373-65-0 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (200.15 mM) | ||||||||
| Ethanol | 33 mg/mL (66.05 mM) | ||||||||||
| Water | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Osimertinib (AZD9291) is an oral, irreversible, and mutant-selective EGFR inhibitor with IC50 of 12.92, 11.44 and 493.8 nM for Exon 19 deletion EGFR, L858R/T790M EGFR, and WT EGFR in LoVo cells, respectively. Phase 3. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | AZD9291 shows significantly more potent inhibition of proliferation in mutant EGFR cell lines compared to wild-type in vitro. [2] |
||||||
| In vivo | AZD9291(5mg/kg p.o.) causes profound regression of tumors across EGFRm+ (PC9) and EGFRm+/T790M (H1975) tumor models with profound inhibition of EGFR phosphorylation and key downstream signaling pathways such as AKT and ERK in vivo. [2] |
||||||
| Features | Orally bioavailable mutant-selective EGFR inhibitor that has been tested in Phase III clinical trials for treatment of Non-Small Cell Lung Cancer. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
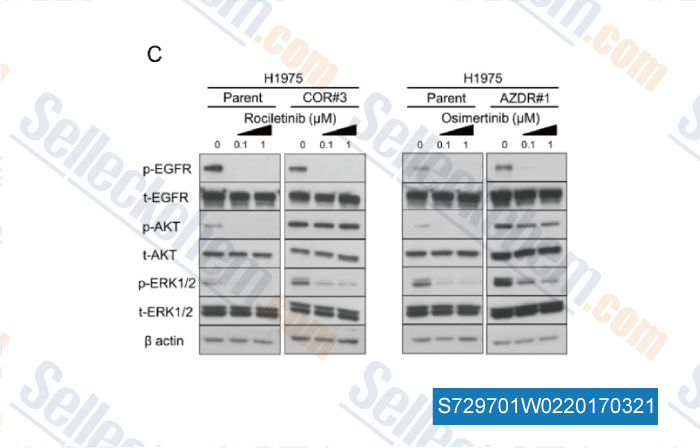
-
, , Cancer Res, 2017, 77(8):2078-2089
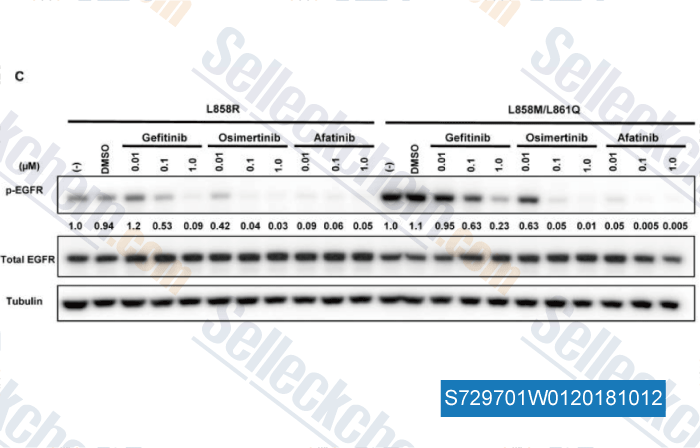
-
Data from [Data independently produced by , , J Thorac Oncol, 2017, 12(5):884-889]
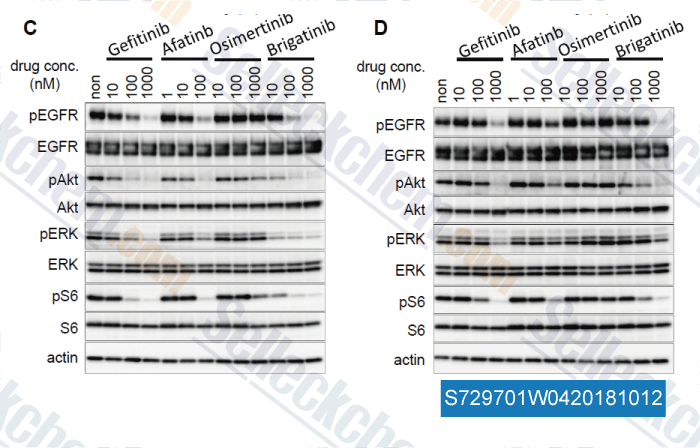
-
Data from [Data independently produced by , , J Thorac Oncol, 2018, 13(7):915-925]
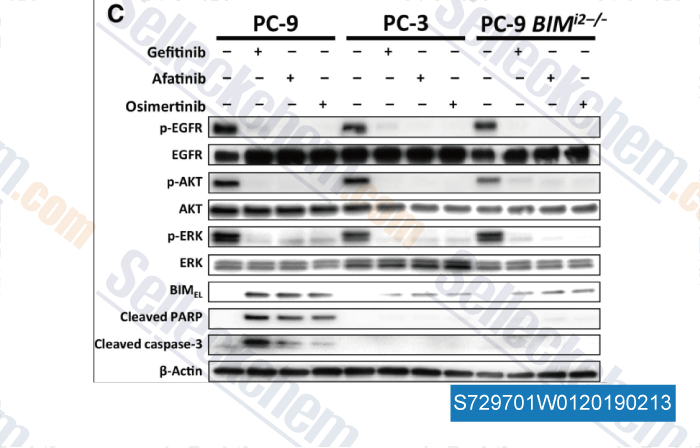
-
Data from [Data independently produced by , , Clin Cancer Res, 2017, 23(12):3139-3149]
Selleck's Osimertinib (AZD9291) has been cited by 416 publications
| Tet methylcytosine dioxygenase 2 (TET2) deficiency elicits EGFR-TKI (tyrosine kinase inhibitors) resistance in non-small cell lung cancer [ Signal Transduct Target Ther, 2024, 9(1):65] | PubMed: 38461173 |
| Comprehensive mutational scanning of EGFR reveals TKI sensitivities of extracellular domain mutants [ Nat Commun, 2024, 15(1):2742] | PubMed: 38548752 |
| Targeting of vulnerabilities of drug-tolerant persisters identified through functional genetics delays tumor relapse [ Cell Rep Med, 2024, 5(3):101471] | PubMed: 38508142 |
| TP53 gain-of-function mutations promote osimertinib resistance via TNF-α-NF-κB signaling in EGFR-mutated lung cancer [ NPJ Precis Oncol, 2024, 8(1):60] | PubMed: 38431700 |
| A destabilizing Y891D mutation in activated EGFR impairs sensitivity to kinase inhibition [ NPJ Precis Oncol, 2024, 8(1):3] | PubMed: 38182677 |
| Mobocertinib in Patients with EGFR Exon 20 Insertion-Positive Non-Small Cell Lung Cancer (MOON): An International Real-World Safety and Efficacy Analysis [ Int J Mol Sci, 2024, 25(7)3992] | PubMed: 38612799 |
| Genetic alterations predict poor efficacy, outcomes and resistance to second-line osimertinib treatment in non-small cell lung cancer [ Am J Cancer Res, 2024, 14(1):33-51] | PubMed: 38323283 |
| The Diagnostic Value of ACSL1, ACSL4, and ACSL5 and the Clinical Potential of an ACSL Inhibitor in Non-Small-Cell Lung Cancer [ Cancers (Basel), 2024, 16(6)1170] | PubMed: 38539505 |
| Revealing underlying regulatory mechanisms of LINC00313 in Osimertinib-resistant LUAD cells by ceRNA network analysis [ Transl Oncol, 2024, 43:101895] | PubMed: 38377935 |
| Non-small cell lung cancer cells with uncommon EGFR exon 19delins variants respond poorly to third-generation EGFR inhibitors [ Transl Oncol, 2024, 39:101834] | PubMed: 38006760 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
