|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C28H21N7OS |
|||
| Molecular Weight | 503.58 | CAS No. | 945595-80-2 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (198.57 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | AMG 900 is a potent and highly selective pan-Aurora kinases inhibitor for Aurora A/B/C with IC50 of 5 nM/4 nM /1 nM. It is >10-fold selective for Aurora kinases than p38α, Tyk2, JNK2, Met and Tie2. Phase 1. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | AMG 900 is a novel class of ATP-competitive phthalazinamine small molecule inhibitors of aurora kinases. In HeLa cells, AMG 900 inhibits autophosphorylation of aurora-A and -B as well as phosphorylation of histone H3 on Ser, a proximal substrate of aurora-B. The predominant cellular response of tumor cells to AMG 900 treatment is aborted cell division without a prolonged mitotic arrest, which ultimately results in cell death. AMG 900 inhibits the proliferation of 26 tumor cell lines, including cell lines resistant and to other aurora kinase inhibitors (AZD1152, MK-0457, and PHA-739358), at low nanomolar concentrations (about 2- 3 nM). Furthermore, AMG 900 is active in an AZD1152-resistant HCT116 variant cell line that harbors an aurora-B mutation (W221L). [1] |
||||||||
| In vivo | Oral administration of AMG 900 blocks the phosphorylation of histone H3 in a dose-dependent manner and significantly inhibited the growth of HCT116 tumor xenografts. AMG 900 is broadly active in multiple xenograft models, including 3 multidrugresistant xenograft models, representing 5 tumor types. [1] AMG 900 exhibits a low-to-moderate clearance and a small volume of distribution. Its terminal elimination half-life ranged from 0.6 to 2.4 hours. AMG 900 is well-absorbed in fasted animals with an oral bioavailability of 31% to 107%. Food intake has an effect on rate (rats) or extent (dogs) of AMG 900 oral absorption. The clearance and volume of distribution at steady state in humans are predicted to be 27.3 mL/h/kg and 93.9 mL/kg, respectively. AMG 900 exhibits acceptable PK properties in preclinical species and is predicted to have low clearance in humans. [2] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
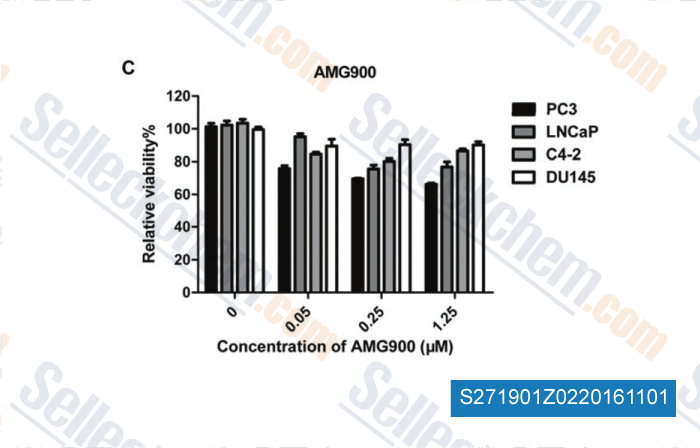
-
, , Am J Transl Res, 2013, 5(3):359-367.
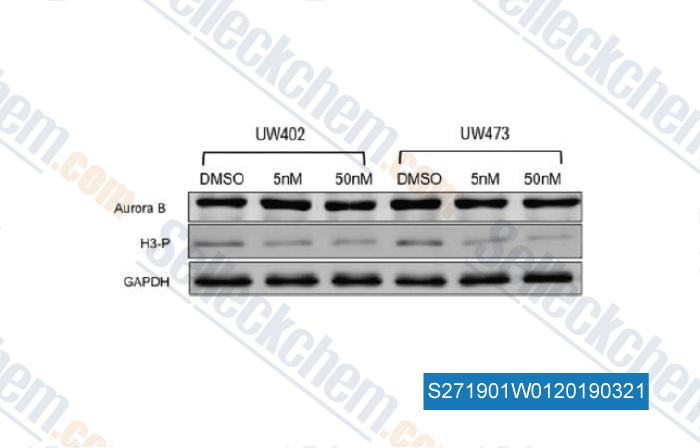
-
Data from [Data independently produced by , , Neurol Res, 2015, 37(8):703-11]
Selleck's AMG-900 has been cited by 22 publications
| DELs enable the development of BRET probes for target engagement studies in cells [ Cell Chem Biol, 2023, 30(8):987-998.e24] | PubMed: 37490918 |
| Poziotinib for EGFR exon 20-mutant NSCLC: Clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity [ Cancer Cell, 2022, 40(7):754-767.e6] | PubMed: 35820397 |
| Primary Cilia Restrain PI3K-AKT Signaling to Orchestrate Human Decidualization [ Int J Mol Sci, 2022, 23(24)15573] | PubMed: 36555215 |
| TubulinTracker, a Novel In Vitro Reporter Assay to Study Intracellular Microtubule Dynamics, Cell Cycle Progression, and Aneugenicity [ Toxicol Sci, 2022, kfac008] | PubMed: 35094094 |
| In vitro human cell-based aneugen molecular mechanism assay [ Environ Mol Mutagen, 2022, 63(3):151-161] | PubMed: 35426156 |
| Cyclin-dependent Kinase 1 and Aurora Kinase choreograph mitotic storage and redistribution of a growth factor receptor [ PLoS Biol, 2021, 19(1):e3001029] | PubMed: 33395410 |
| Inhibition of Aurora kinase A activity enhances the antitumor response of beta-catenin blockade in human adrenocortical cancer cells [ Mol Cell Endocrinol, 2021, 528:111243] | PubMed: 33716050 |
| Experiments in the EpiDerm™ 3D Skin in Vitro Model and Minipigs in Vivo Indicate Comparatively Lower in Vivo Skin Sensitivity of Topically Applied Aneugenic Compounds [ Toxicol Sci, 2021, kfaa189] | PubMed: 33481035 |
| Biomarkers of DNA Damage Response Improves In Vitro Micronucleus Assays by Revealing Genotoxic Mode of Action and Reducing the Occurrence of Irrelevant Positive Results [ Mutagenesis, 2021, geab039] | PubMed: 34718711 |
| Prolonged unfolded protein reaction is involved in the induction of chronic myeloid leukemia cell death upon oprozomib treatment [ Cancer Sci, 2020, 112(1):133-143] | PubMed: 33067904 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
