|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C21H16N6O2S2 |
||||||
| Molecular Weight | 448.52 | CAS No. | 956958-53-5 | ||||
| Solubility (25°C)* | In vitro | DMSO | 90 mg/mL (200.65 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | XL147 analogue (SAR245408) is a selective and reversible class I PI3K inhibitor for PI3Kα/δ/γ with IC50 of 39 nM/36 nM/23 nM in cell-free assays, less potent to PI3Kβ. XL147 analogue induces apoptosis. Phase 1/2. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | XL147 inhibits class I PI3K isoforms in an ATP-competitive manner. In a panel of HER2-overexpressing human breast cancer cell lines, treatment with XL147 abrogates AKT and S6 phosphorylation but also induces the expression and phosphorylation of HER3 and other RTKs. In HER2+ cells, phosphorylation of HER3 is maintained by the HER2 tyrosine kinase, leading to partial recovery of phosphorylated AKT (pAKT) and thereby limiting the antitumor action of XL 147. In addition, knockdown of HER3 or treatment with the anti-HER2 agents trastuzumab or lapatinib sensitizes HER2+ breast cancer cells to XL147 in vitro and in vivo. Treatment with XL147 inhibits the monolayer growth of all tested cell lines, including BT474, HCC1937 et al. in a dose-dependent manner. The main effect of XL147 is inhibition of cell proliferation. XL147 induces cell death at the concentration of 20 μM. Treatment with XL147 leads to dose-dependent inhibition of PI3K. Consistent with the inhibition of cell proliferation, XL147 induces a reduction in cyclin D1 and pRB and an increase in levels of the CDK inhibitor p27KIPI but no detectable change in levels off total or cleaved poly (ADP-ribose) polymerase (PARP). Treatment with XL147 leads to a dose-dependent reduction in pAKTS473/T308 and pS6S240/244. Surprisingly, XL147 also triggers up-regulation of total HER3 and/or pHER3Y1289 levels. In HER2-overexpressing cells, inhibition of PI3K is followed by up-regulation of expression and phosphorylation of multiple receptor tyrosine kinases, including HER3. Knockdown of FoxO1 and FoxO3a transcription factors prevents the induction of HER3, InsR, IGF1R, and FGFR2 mRNAs upon inhibition of PI3K. In HER2+ cells, knockdown of HER3 with siRNA or cotreatment with the HER2 inhibitors trastuzumab or lapatinib enhances XL147-induced cell death and inhibition of pAKT and pS6. [2] | ||||||||
| In vivo | Athymic mice with BT474 xenografts are randomly treated with XL147, lapatinib, trastuzumab, or XL147 plus each HER2 antagonist. Each monotherapy significantly inhibtis tumor growth with trastuzumab being the only agent that induced a complete tumor regression in one of eight mice. Both combinations are superior to the respective drugs given alone. Notably, the combination of trastuzumab and XL147, but not lapatinib and XL147, induces a complete tumor response in three of eight mice. There is no marked drug-related toxicity in any of the treatment arms. The combination of XL147 plus trastuzumab prevents pHER3 more potently than any of the other treatments. In good agreement with differences in tumor growth among treatment arms, nuclear pAKT is lower in tumors treated with XL147 plus lapatinib or XL147 plus trastuzumab compared with tumors treated with single agents. Of all three single drugs, XL147 is the only one shown statistically to repress nuclear pAKT levels. There are no detectable changes in cytoplasmic pAKT levels. Combined inhibition of HER2 and PI3K in HER2-dependent xenografts is required to maximally inhibit signaling output of the PI3K/AKT pathway. [2] |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
|
Customer Product Validation
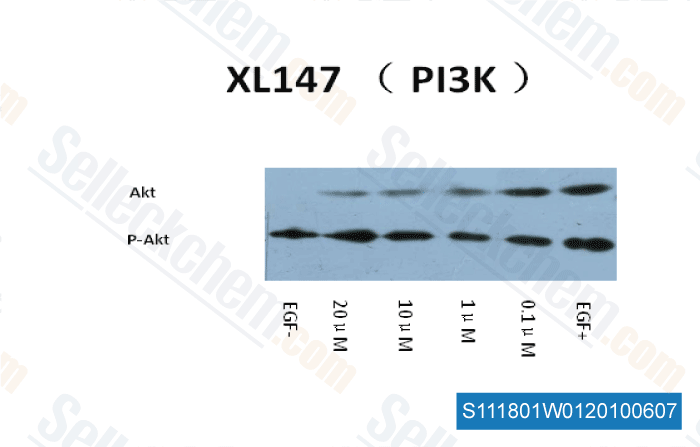
-
Data independently produced by Dr. Zhang of Tianjin Medical University ,
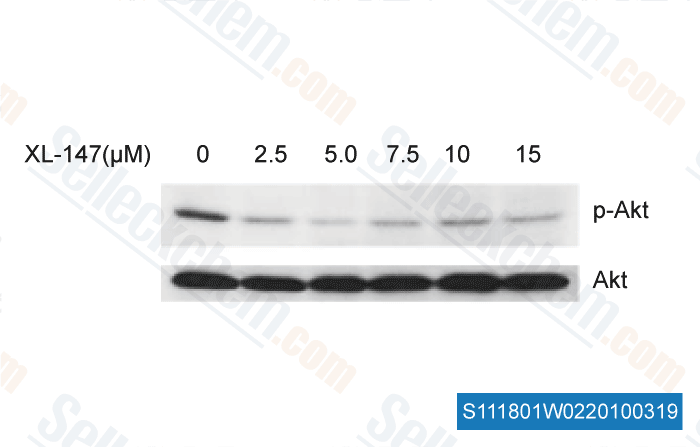
-
, , Dr. Chunrong Yu of RoswelI Park Cancer Institute
Selleck's XL147 analogue has been cited by 12 publications
| Establishment and Characterization of NCC-PMP1-C1: A Novel Patient-Derived Cell Line of Metastatic Pseudomyxoma Peritonei [ J Pers Med, 2022, 12(2)258] | PubMed: 35207746 |
| Establishment and characterization of NCC-UPS4-C1: a novel cell line of undifferentiated pleomorphic sarcoma from a patient with Li-Fraumeni syndrome [ Hum Cell, 2022, 10.1007/s13577-022-00671-y] | PubMed: 35118583 |
| Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00579-z] | PubMed: 34304386 |
| Establishment and characterization of the NCC-GCTB4-C1 cell line: a novel patient-derived cell line from giant cell tumor of bone [ Hum Cell, 2021, 10.1007/s13577-021-00639-4] | PubMed: 34731453 |
| Establishment and characterization of NCC-MFS4-C1: a novel patient-derived cell line of myxofibrosarcoma [ Hum Cell, 2021, 34(6):1911-1918] | PubMed: 34383271 |
| Establishment and characterization of NCC-MFS2-C1: a novel patient-derived cancer cell line of myxofibrosarcoma [ Hum Cell, 2020, 10.1007/s13577-020-00420-z] | PubMed: 32870449 |
| hMENA11a contributes to HER3-mediated resistance to PI3K inhibitors in HER2-overexpressing breast cancer cells. [Trono P,et al. Oncogene, 2015, 10.1038/onc.2015.143] | PubMed: 25961924 |
| Quantitative optical imaging of primary tumor organoid metabolism predicts drug response in breast cancer [Walsh AJ, et al Cancer Res, 2014, 74(18):5184-94] | PubMed: 25100563 |
| Deciphering combinations of PI3K/AKT/mTOR pathway drugs augmenting anti-angiogenic efficacy in vivo [ PLoS One, 2014, 9(8):e105280] | PubMed: 25144531 |
| Deciphering Combinations of PI3K/AKT/mTOR Pathway Drugs Augmenting Anti-Angiogenic Efficacy In Vivo [Sasore T, et al PLoS One, 2014, 9(8):e105280] | PubMed: 25144531 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
