|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C17H22N2O3 |
|||
| Molecular Weight | 302.4 | CAS No. | 58880-19-6 | |
| Solubility (25°C)* | In vitro | DMSO | 60 mg/mL (198.41 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | TSA (Trichostatin A) is an HDAC inhibitor with IC50 of ~1.8 nM in cell-free assays. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Trichostatin A inhibits the proliferation of eight breast carcinoma cell lines including MCF-7, T-47D, ZR-75-1, BT-474, MDA-MB-231, MDA-MB-453, CAL 51, and SK-BR-3 with mean IC50 of 124.4 nM (range, 26.4-308.1 nM), with more potency against cell lines that express ERα than the ERα-negative cell lines. Trichostatin A inhibits HDAC activity similarly in all the breast cancer cell lines with mean IC50 of 2.4 nM (range, 0.6-2.6 nM), and results in pronounced histone H4 hyperacetylation. [1] Unlike Trapoxin (TPX) and Chlamydocin which potently inhibit HDAC1 or HDAC4 but not HDAC6, Trichostatin A inhibits these HDACs to a similar extent with IC50 of 6 nM, 38 nM, and 8.6 nM, respectively. [2] Trichostatin A (100 ng/mL) treatment induces the expression of transforming growth factor β type II receptor (TβRII) in MIA PaCa-2 cells through the recruitment of p300 and PCAF into a Sp1-NF-Y HDAC complex that binds the DNA element of TβRII promoter, which is associated with a concomitant acetylation of Sp1 and an overall decrease in the amount of HDAC associated with the complex. [4] | ||
| In vivo | Administration of Trichostatin A at 0.5 mg/kg for 4 weeks displays potent antitumor activity in the N-methyl-N-nitrosourea carcinogen-induced rat mammary carcinoma model, without any measurable toxicity at doses up to 5 mg/kg. [1] Single intraperitoneal doses of 10 mg/kg Trichostatin A in nontransgenic and spinal muscular atrophy (SMA) model mice results in increased levels of acetylated H3 and H4 histones and modest increases in survival motor neuron (SMN) gene expression. Administration of Trichostatin A at 10 mg/kg/day improves survival, attenuates weight loss, and enhances motor behavior in the SMA model mice. [5] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
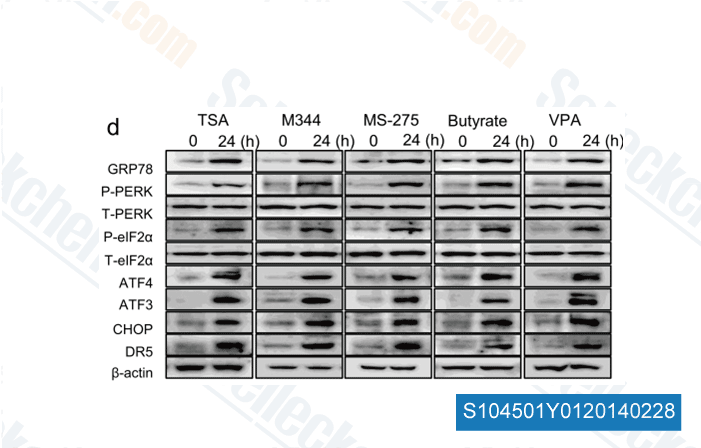
-
Data from [Biochem Biophys Res Commun, 2014, 10.1016/j.bbrc.2014.01.184]
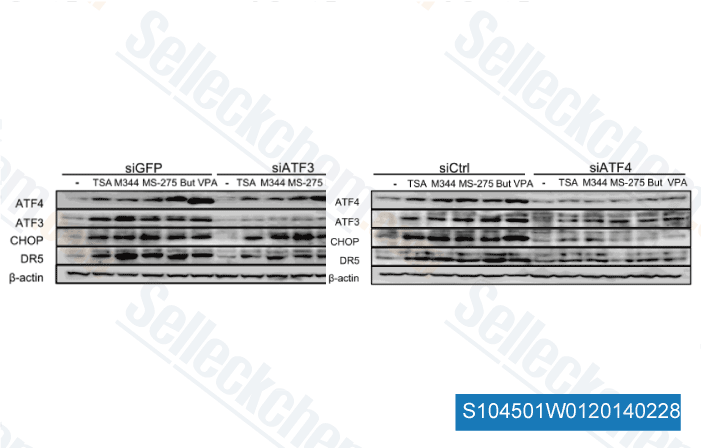
-
Data from [Biochem Biophys Res Commun, 2014, 10.1016/j.bbrc.2014.01.184]
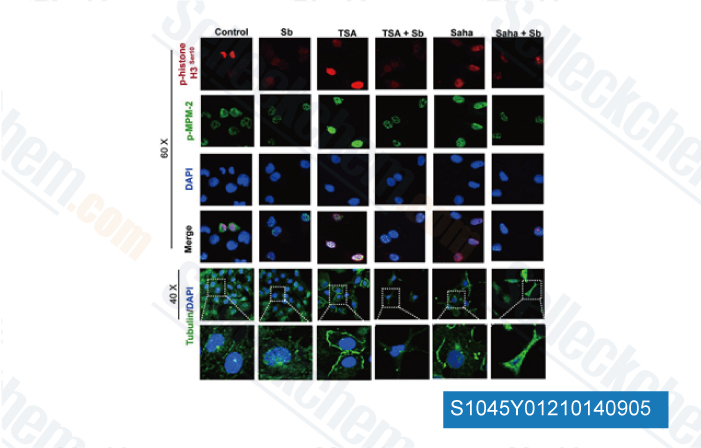
-
Data from [Epigenetics, 2012, 7(10):1161-72]
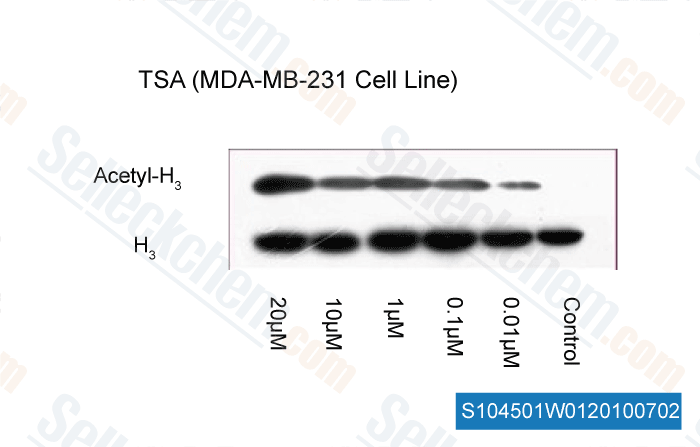
-
, 2010, Dr. Zhang of Tianjin Medical University
Selleck's TSA (Trichostatin A) has been cited by 274 publications
| Glucocorticoids increase adiposity by stimulating Krüppel-like factor 9 expression in macrophages [ Nat Commun, 2024, 15(1):1190] | PubMed: 38331933 |
| Unveiling the mechanism of broad-spectrum blast resistance in rice: The collaborative role of transcription factor OsGRAS30 and histone deacetylase OsHDAC1 [ Plant Biotechnol J, 2024, 10.1111/pbi.14299] | PubMed: 38294722 |
| The ARID1A-METTL3-m6A axis ensures effective RNase H1-mediated resolution of R-loops and genome stability [ Cell Rep, 2024, 43(2):113779] | PubMed: 38358891 |
| GCN5 mediates DNA-PKcs crotonylation for DNA double-strand break repair and determining cancer radiosensitivity [ Br J Cancer, 2024, 10.1038/s41416-024-02636-4] | PubMed: 38575732 |
| Propionate functions as a feeding-state-dependent regulatory metabolite to counter pro-inflammatory signaling linked to nutrient load and obesity [ J Leukoc Biol, 2024, qiae006] | PubMed: 38207130 |
| Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative [ Pharmaceutics, 2024, 16(1)126] | PubMed: 38258135 |
| HDAC1/2/3 are major histone desuccinylases critical for promoter desuccinylation [ Cell Discov, 2023, 9(1):85] | PubMed: 37580347 |
| SCARB2 drives hepatocellular carcinoma tumor initiating cells via enhanced MYC transcriptional activity [ Nat Commun, 2023, 14(1):5917] | PubMed: 37739936 |
| SCARB2 drives hepatocellular carcinoma tumor initiating cells via enhanced MYC transcriptional activity [ Nat Commun, 2023, 14(1):5917] | PubMed: 37739936 |
| Histone 4 lysine 5/12 acetylation enables developmental plasticity of Pristionchus mouth form [ Nat Commun, 2023, 14(1):2095] | PubMed: 37055396 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
