|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C8H12N4O5 |
|||
| Molecular Weight | 244.20864 | CAS No. | 36791-04-5 | |
| Solubility (25°C)* | In vitro | DMSO | 49 mg/mL (200.64 mM) | |
| Water | 49 mg/mL (200.64 mM) | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Ribavirin (NSC-163039, ICN-1229, RTCA, Tribavirin), a synthetic guanosine analogue, possesses a broad spectrum of activity against DNA and RNA viruses. |
|---|---|
| In vitro | Ribavirin significantly reduces the efficiency with which progeny subgenomic replicons transfect new cells in the replicon system, although ribavirin has little effect on levels of HCV replication. Ribavirin increases the mutation frequency of HCV, with the highest rates of mutations being found in the NS5A-encoding region. Ribavirin enhances TH1 while inhibiting T 2 cytokine production by stimulated T cells. [1] Ribavirin shows antiviral activity against a variety of RNA viruses and is used in combination with interferon-alpha to treat hepatitis C virus infection. Ribavirin reduces infectious poliovirus production to as little as 0. 00001% in cell culture. [2] Ribavirin's antiviral activity is exerted directly through lethal mutagenesis of the viral genetic material. [3] Ribavirin markedly reduces viral-induced parameters of macrophage activation at physiologic concentrations (up to 500 mg/mL). Ribavirin inhibits the production of IL-4 by Th2 cells, whereas it does not diminish the production of IFN-gamma in Th1 cells. [4] Ribavirin exhibits antiviral activity against a broad range of both DNA and RNA viruses in vitro. Ribavirin is a cytostatic agent and causes a reduction in synthesis of DNA, RNA and proteins in exposed cells. Ribavirin is thought to induce a switch in T-helper cell phenotype from type 2 to type 1. [5] |
Protocol (from reference)
References
Customer Product Validation
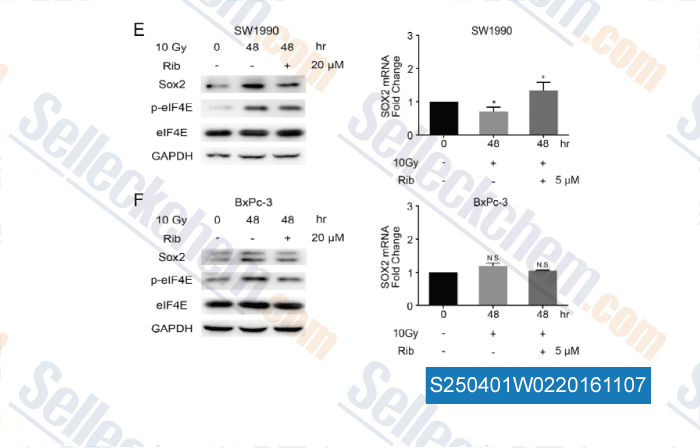
-
, , Cancer Lett, 2016, 375(1):31-8.
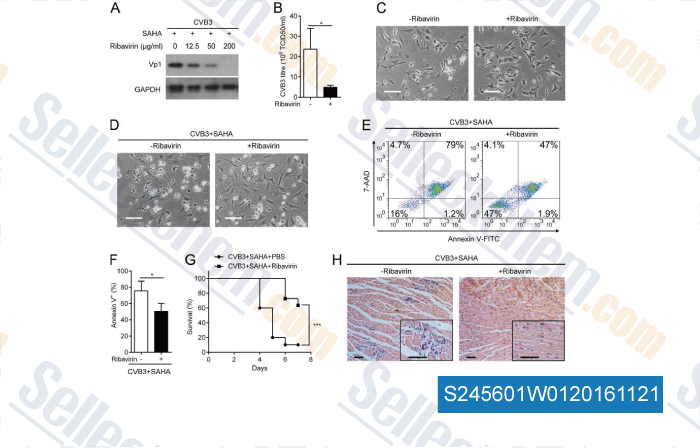
-
Data from [Data independently produced by , , J Virol, 2015, 89(20):10512-23.]
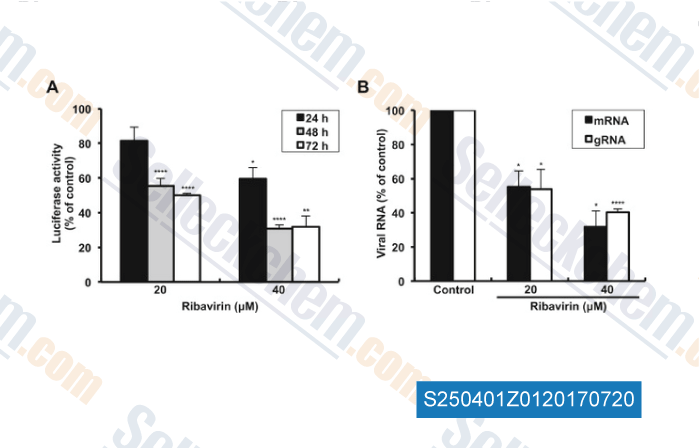
-
Data from [Data independently produced by , , Antiviral Res, 2017, 143:237-245]
Selleck's Ribavirin (ICN-1229) has been cited by 21 publications
| Biological and Structural Analyses of New Potent Allosteric Inhibitors of HIV-1 Integrase [ Antimicrob Agents Chemother, 2023, 67(7):e0046223] | PubMed: 37310224 |
| RECOVER identifies synergistic drug combinations in vitro through sequential model optimization [ Cell Rep Methods, 2023, 3(10):100599] | PubMed: 37797618 |
| An anti-influenza A virus microbial metabolite acts by degrading viral endonuclease PA [ Nat Commun, 2022, 13(1):2079] | PubMed: 35440123 |
| Revisiting the Mongolian Gerbil Model for Hepatitis E Virus by Reverse Genetics [ Microbiol Spectr, 2022, e0219321] | PubMed: 35230152 |
| 2-((1H-indol-3-yl)thio)-N-phenyl-acetamides: SARS-CoV-2 RNA-dependent RNA polymerase inhibitors [ Antiviral Res, 2021, 196:105209] | PubMed: 34801588 |
| A cell-based assay to discover inhibitors of SARS-CoV-2 RNA dependent RNA polymerase [ Antiviral Res, 2021, S0166-3542(21)00068-1] | PubMed: 33894278 |
| Pre-Senescence Induction in Hepatoma Cells Favors Hepatitis C Virus Replication and Can Be Used in Exploring Antiviral Potential of Histone Deacetylase Inhibitors [ Int J Mol Sci, 2021, 22(9)4559] | PubMed: 33925399 |
| The NF-κB inhibitor, SC75741, is a novel antiviral against emerging tick-borne bandaviruses [ Antiviral Res, 2020, 185:104993] | PubMed: 33296695 |
| High-Throughput Screening of an FDA-Approved Drug Library Identifies Inhibitors against Arenaviruses and SARS-CoV-2 [ ACS Infect Dis, 2020, acsinfecdis.0c00486] | PubMed: 33183004 |
| Project IDentif.AI: Harnessing Artificial Intelligence to Rapidly Optimize Combination Therapy Development for Infectious Disease Intervention [ Adv Ther (Weinh), 2020, 2000034] | PubMed: 32838027 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
