|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C50H46CaF2N2O8 |
|||
| Molecular Weight | 880.98 | CAS No. | 147526-32-7 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (113.5 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Pitavastatin calcium, a novel member of the medication class of statins, is a calcium salt formulation of pitavastatin which is a highly effective HMG-CoA reductase inhibitor. Pitavastatin Calcium attenuates AGEs-induced mitophagy via inhibition of ROS generation. Pitavastatin Calcium induces autophagy and apoptosis. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Pitavastatin significantly reduces both intracellular levels and synthesis of cholesterol esters. Pitavastatin is found to enhance LDL-receptor expression in vitro, as well as the amount of LDL binding to the LDL-receptor. Pitavastatin also exhibits more potent induction of LDL receptor mRNA expression compared with simvastatin and atorvastatin. Pitavastatin has many pleiotropic effects in vitro and in vivo, including deterring progression of atherosclerosis via inhibition of thromboxane synthesis, inhibition of migration/proliferation of vascular smooth muscle cells induced by angiotensin II, and stabilization of atherosclerotic plaque. [1] Pitavastatin is able to activate PPARα and induce HDL apoA-I through inducing inhibition of the Rho-signaling pathway. [2] Pitavastatin (1 μM) treatment for 48 h is able to enhances bone morphogenetic protein-2 BMP-2 (2.5-fold) and osteocalcin (10-fold) expression by inhibition of Rho-associated kinase in human osteoblasts[3]. Pitavastatin inhibits growth and colony formation of liver cancer Huh-7 cells and SMMC7721 cells. It induces arrest of liver cancer cells at the G1 phase. Increased proportion of sub-G1 cells is observed after pitavastatin treatment. Pitavastatin promotes caspase-9 cleavage and caspase-3 cleavage in liver cancer cells. Pitavastatin could regulate NF-κB and anti-inflammation in hepatocellular carcinoma cells. Pitavastatin could induce autophagic cell death in glioma cells and promote sensitivity of cells to radiotherapy. It could inhibit cell proliferation and induce cell apoptosis in cholangiocarcinoma cells as well[5]. | ||
| In vivo | Pitavastatin decreases the tumor growth and improved the survival of tumor-bearing mice[5]. Pitavastatin exerts a protective effect on dilated cardiomyopathy possibly through down-regulating the circulating and local RAS, followed by inhibition of PKCb2 phosphorylation, and consequently promoting the phosphorylation of PLB as well as the activity and the expressions of SERCA2a and RyR2, whereby heart function is preserved in the development of DCM[6]. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
Customer Product Validation
-
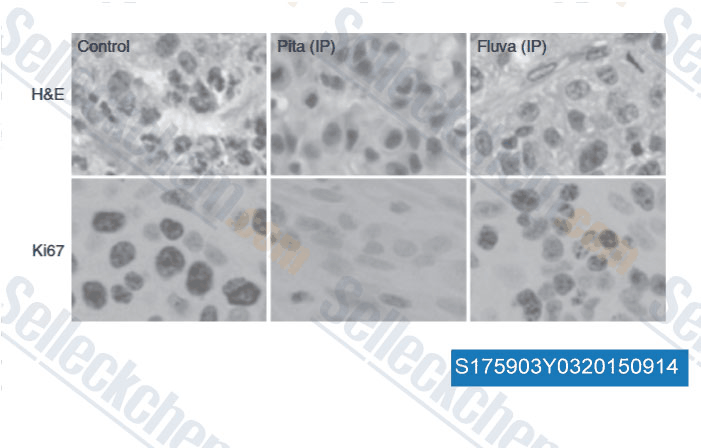
Data from [Data independently produced by , , Br J Cancer, 2014, 111(8): 1562-71 ]
-
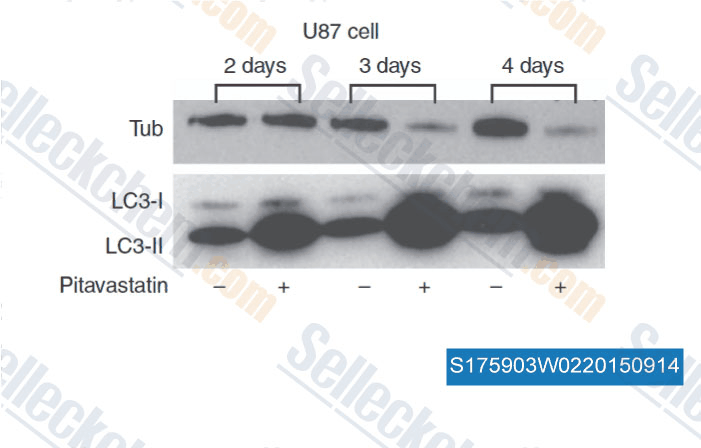
Data from [Data independently produced by , , Br J Cancer, 2014, 111(8): 1562-71 ]
-
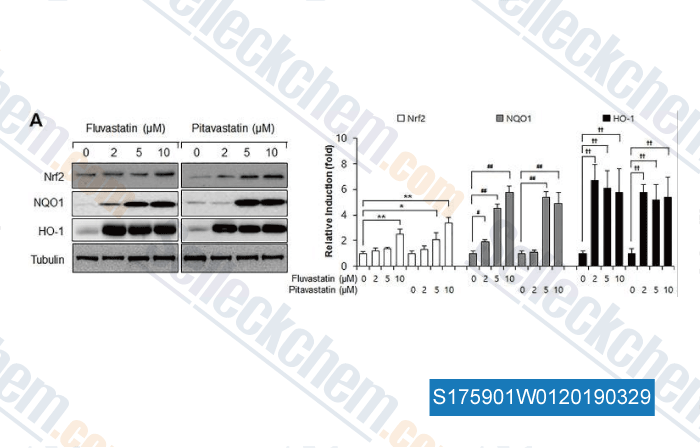
Data from [Data independently produced by , , PLoS One, 2017, 12(5):e0178278]
Selleck's Pitavastatin calcium has been cited by 23 publications
| Gene regulatory network topology governs resistance and treatment escape in glioma stem-like cells [ bioRxiv, 2024, 2024.02.02.578510] | PubMed: 38370784 |
| Cholesterol biosynthesis inhibition synergizes with AKT inhibitors in triple-negative breast cancer [ bioRxiv, 2024, 10.1101/2024.01.16.575899] | PubMed: none |
| Epigenetic suppression of PGC1α (PPARGC1A) causes collateral sensitivity to HMGCR-inhibitors within BRAF-treatment resistant melanomas [ Nat Commun, 2023, 14(1):3251] | PubMed: 37277330 |
| Caffeine Supplementation and FOXM1 Inhibition Enhance the Antitumor Effect of Statins in Neuroblastoma [ Cancer Res, 2023, 83(13):2248-2261] | PubMed: 37057874 |
| Phenotypic screening platform identifies statins as enhancers of immune cell-induced cancer cell death [ BMC Cancer, 2023, 23(1):164] | PubMed: 36803614 |
| Pitavastatin Induces Apoptosis of Cutaneous Squamous Cell Carcinoma Cells through Geranylgeranyl Pyrophosphate-Dependent c-Jun N-Terminal Kinase Activation [ Ann Dermatol, 2023, 35(2):116-123] | PubMed: 37041705 |
| Statin prevents cancer development in chronic inflammation by blocking interleukin 33 expression [ Res Sq, 2023, rs.3.rs-2318750] | PubMed: 36711701 |
| Follicular lymphoma-associated mutations in the V-ATPase chaperone VMA21 activate autophagy creating a targetable dependency [ Autophagy, 2022, 1-19] | PubMed: 35287545 |
| The Antineoplastic Effect of Carboplatin Is Potentiated by Combination with Pitavastatin or Metformin in a Chemoresistant High-Grade Serous Carcinoma Cell Line [ Int J Mol Sci, 2022, 24(1)97] | PubMed: 36613537 |
| Pitavastatin and Ivermectin Enhance the Efficacy of Paclitaxel in Chemoresistant High-Grade Serous Carcinoma [ Cancers (Basel), 2022, 14(18)4357] | PubMed: 36139522 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
