|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C10H15N5.HCl |
|||
| Molecular Weight | 241.72 | CAS No. | 834-28-6 | |
| Solubility (25°C)* | In vitro | DMSO | 48 mg/mL (198.57 mM) | |
| Water | 48 mg/mL (198.57 mM) | |||
| Ethanol | 12 mg/mL (49.64 mM) | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Phenformin HCl is a hydrochloride salt of phenformin that is an anti-diabetic drug from the biguanide class. It activates AMPK, increasing activity and phosphorylation. | |
|---|---|---|
| Targets |
|
|
| In vitro | Phenformin stimulates the phosphorylation and activation of AMPKalpha1 and AMPKalpha2 without altering LKB1 activity. [1] Phenformin increases AMPK activity and phosphorylation in the isolated heart, the increase in AMPK activity is always preceded by and correlated with increased cytosolic [AMP]. [2] Phenformin is a 50-fold more potent inhibitor of mitochondrial complex I than metformin. Phenformin robustly induces apoptosis in LKB1 deficient NSCLC cell lines. Phenformin at 2 mM similarly induces AMPK signaling as shown by increased P-AMPK and P-Raptor levels. Phenformin induces higher levels of cellular stress, triggering induction of P-Ser51 eIF2α and its downstream target CHOP, and markers of apoptosis at later times. Phenformin induces a significant increase in survival and therapeutic response in KLluc mice following long-term treatment. [3] Phenformin and AICAR increases AMPK activity in H441 cells in a dose-dependent fashion, stimulating the kinase maximally at 5-10 mm and 2 mm, respectively. Phenformin significantly decreases basal ion transport (measured as short circuit current) across H441 monolayers by approximately 50% compared with that of controls. Phenformin and AICAR significantly reduce amiloride-sensitive transepithelial Na+ transport compared with controls. Phenformin and AICAR suppress amiloride-sensitive Na+ transport across H441 cells via a pathway that includes activation of AMPK and inhibition of both apical Na+ entry through ENaC and basolateral Na+ extrusion via the Na+,K+-ATPase. [4] Phenformin-treated rats reveals a tendency towards a decrease in blood insulin level (radioimmunoassay). [5] | |
| In vivo | Phenformin also increases levels of P-eIF2α and its target BiP/Grp78 in normal lung as well as in lung tumors of mice. [3] |
Protocol (from reference)
References
Customer Product Validation
-
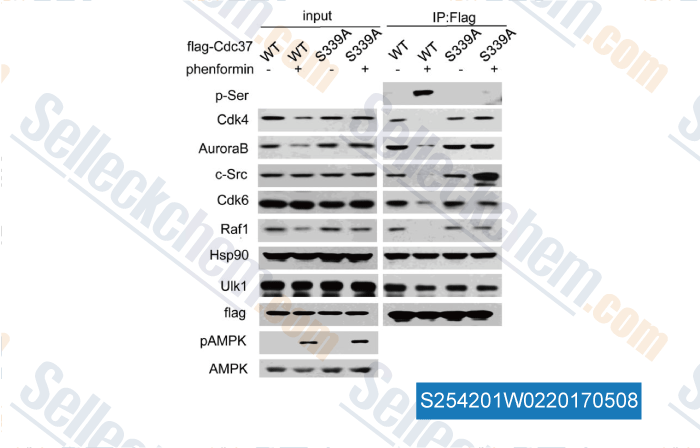
Data from [Data independently produced by , , J Biol Chem, 2017, 292(7):2830-2841]
-
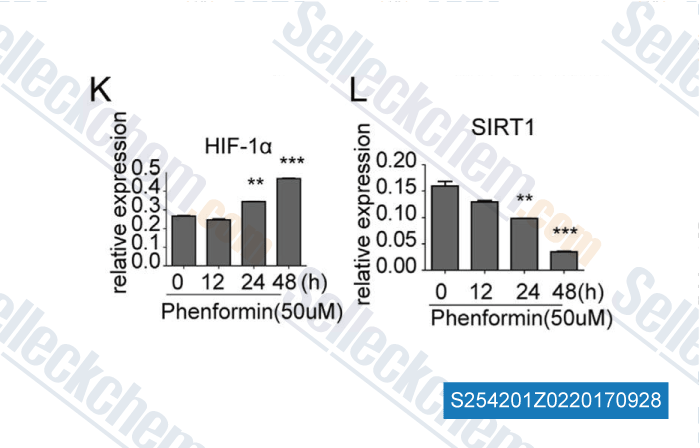
Data from [Data independently produced by , , Biochem Biophys Res Commun, 2016, 470(2):453-459]
Selleck's Phenformin HCl has been cited by 9 publications
| Quiescence enables unrestricted cell fate in naive embryonic stem cells [ Nat Commun, 2024, 15(1):1721] | PubMed: 38409226 |
| Identification of a novel m6A-related lncRNAs signature and immunotherapeutic drug sensitivity in pancreatic adenocarcinoma [ BMC Cancer, 2024, 24(1):116] | PubMed: 38262966 |
| Screening Health-Promoting Compounds for Their Capacity to Induce the Activity of FOXO3 [ J Gerontol A Biol Sci Med Sci, 2022, 77-8:1485-1493] | PubMed: 34508571 |
| Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool [ cell, 2019, 178(5):1102-1114] | PubMed: 31442403 |
| Liquid biopsy-based single-cell metabolic phenotyping of lung cancer patients for informative diagnostics. [ Nat Commun, 2019, 10(1):3856] | PubMed: 31451693 |
| SPARC Inhibits Metabolic Plasticity in Ovarian Cancer [ Cancers (Basel), 2018, 10(10)E385] | PubMed: 30332737 |
| [ Oncotarget, 2017, ] | PubMed: 28938614 |
| Serine/Threonine Kinase Unc-51-like Kinase-1 (Ulk1) Phosphorylates the Co-chaperone Cell Division Cycle Protein 37 (Cdc37) and Thereby Disrupts the Stability of Cdc37 Client Proteins. [Li R, et al. J Biol Chem, 2017, 292(7):2830-2841] | PubMed: 28073914 |
| The epigenetic regulation of HIF-1α by SIRT1 in MPP(+) treated SH-SY5Y cells. [Dong SY, et al. Biochem Biophys Res Commun, 2016, 470(2):453-9] | PubMed: 26768367 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
