|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C53H83NO14 |
|||
| Molecular Weight | 958.22 | CAS No. | 159351-69-6 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (104.36 mM) | |
| Ethanol | 100 mg/mL (104.36 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Everolimus is an mTOR inhibitor of FKBP12 with IC50 of 1.6-2.4 nM in a cell-free assay. Everolimus induces cell apoptosis and autophagy and inhibits tumor cells proliferation. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Everolimus exhibits the immunosuppressive activity which is comparable to that of rapamycin. Everolimus competes with immobilized FK 506 for binding to biotinylated FKBP12 and shows the inhibitory effect on a two-way MLR performed with spleen cells from BALB/c and CBA mice with IC50 of 0.12-1.8 nM. [1] Everolimus also shows antiangiogenic/vascular effects in VEGF-induced HUVEC proliferation with IC50 of 0.12 nM and bFGF-induced HUVEC proliferation with IC50 of 0.8 nM, respectively. [2] A recent study shows that Everolimus shows a dose-dependent inhibitory effects on both the total cells and the stem cells from the BT474 cell line and the primary breast cancer cells with IC50 of 156 nM in total cells of primary breast cancer cells and 71 nM in total cells of BT474 cells. In addition, combination treatment with Everolimus and trastuzumab produces the significantly increased inhibition on the growth of cancer stem cells with the inhibition rate increased by more than 50 %. [3] | ||||
| In vivo | Everolimus (0.1 to 10 mg/kg) dose-dependently inhibits growth of the primary (ear) and lymph node metastases of B16/BL6 melanoma, with decreased total number of vessels and reduced mature vessels. [2] In a xenograft animal model of BT474 stem cells, Everolimus shows significant reductions in mean tumor sizes (590.6 mm3), compared to the control group with a tumor size of 698 mm3. Furthermore, combination treatment with Everolimus and trastuzumab significantly decreases the xenograft tumor size (410.8 mm3) more than Everolimus treatment alone. [3] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[3] |
|
| Animal Study:[3] |
|
References
Customer Product Validation
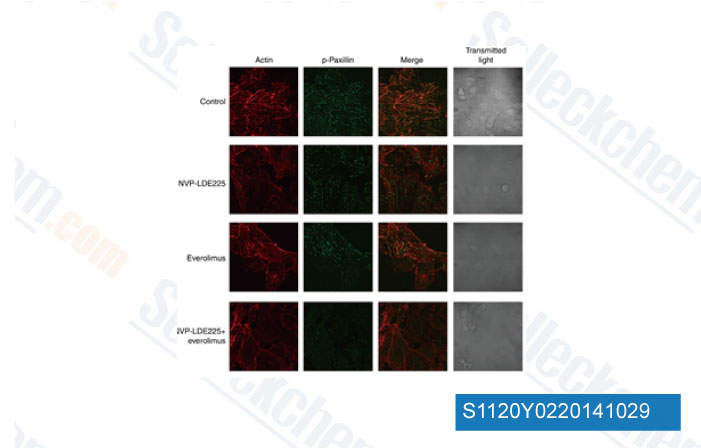
-
Data from [Data independently produced by Br J Cancer, 2014, 111(6), 1168-79]
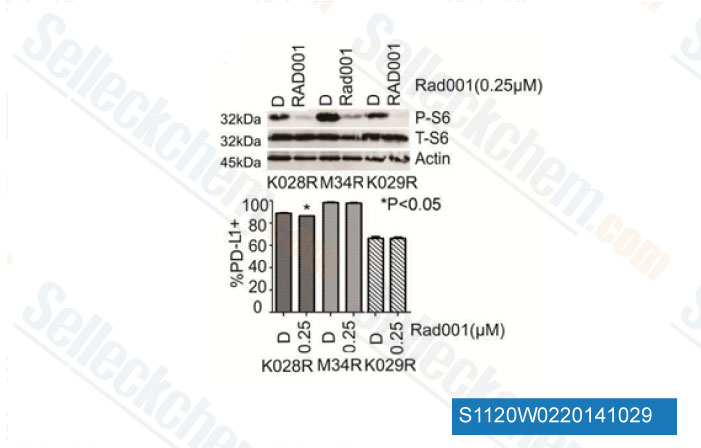
-
Data from [Data independently produced by Clin Cancer Res, 2013, 19(3),598-609]
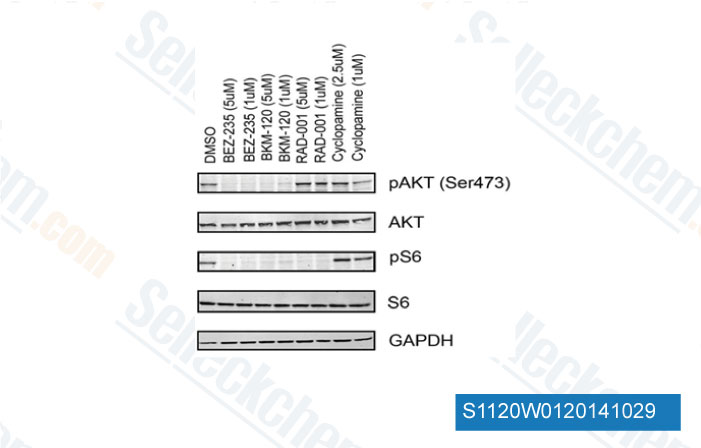
-
Data from [Data independently produced by Cancer Cell, 2012, 21(2), 155-67]
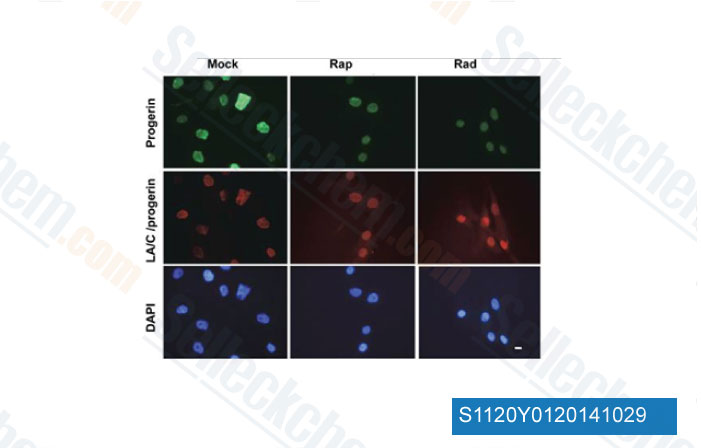
-
Data from [Data independently produced by Aging (Albany NY), 2012, 4(2), 119-32]
Selleck's Everolimus has been cited by 848 publications
| Human fetal brain self-organizes into long-term expanding organoids [ Cell, 2024, 187(3):712-732.e38] | PubMed: 38194967 |
| Altered CELF4 splicing factor enhances pancreatic neuroendocrine tumors aggressiveness influencing mTOR and everolimus response [ Mol Ther Nucleic Acids, 2024, 35(1):102090] | PubMed: 38187140 |
| NF1 deficiency drives metabolic reprogramming in ER+ breast cancer [ Mol Metab, 2024, 80:101876] | PubMed: 38216123 |
| Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness [ Cancer Cell, 2023, 41(1):139-163.e17] | PubMed: 36563681 |
| Metabolic support by macrophages sustains colonic epithelial homeostasis [ Cell Metab, 2023, S1550-4131(23)00341-8] | PubMed: 37804836 |
| Cellular mechanisms of heterogeneity in NF2-mutant schwannoma [ Nat Commun, 2023, 14(1):1559] | PubMed: 36944680 |
| N-acetylcysteine overcomes NF1 loss-driven resistance to PI3Kα inhibition in breast cancer [ Cell Rep Med, 2023, 4(4):101002] | PubMed: 37044095 |
| HOXC6 drives a therapeutically targetable pancreatic cancer growth and metastasis pathway by regulating MSK1 and PPP2R2B [ Cell Rep Med, 2023, 10.1016/j.xcrm.2023.101285] | PubMed: 37951219 |
| Inhibiting the glycerophosphodiesterase EDI3 in ER-HER2+ breast cancer cells resistant to HER2-targeted therapy reduces viability and tumour growth [ J Exp Clin Cancer Res, 2023, 42(1):25] | PubMed: 36670508 |
| ZDHHC2-Mediated AGK Palmitoylation Activates AKT-mTOR Signaling to Reduce Sunitinib Sensitivity in Renal Cell Carcinoma [ Cancer Res, 2023, 83(12):2034-2051] | PubMed: 37078777 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
