|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C21H19ClN4O4 |
||||||||||
| Molecular Weight | 426.85 | CAS No. | 417716-92-8 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 21 mg/mL (49.19 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Lenvatinib is a multi-target inhibitor, mostly for VEGFR2(KDR)/VEGFR3(Flt-4) with IC50 of 4 nM/5.2 nM, less potent against VEGFR1/Flt-1, ~10-fold more selective for VEGFR2/3 against FGFR1, PDGFRα/β in cell-free assays. Lenvatinib (E7080) also inhibits FGFR1-4, PDGFR, Kit (c-Kit), RET (c-RET), and shows potent antitumor activities. Phase 3. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
|||||||||||
| In vitro | E7080, as a potent inhibitor of in vitro angiogenesis, shows a significantly inhibitory effect on VEGF/KDR and SCF/Kit signaling. According to the in vitro receptor tyrosine and serine/threonine kinase assays, E7080 inhibits Flt-1, KDR, Flt-4 with IC50 of 22, 4.0 and 5.2 nM, respectively. In addition to these kinases, E7080 also inhibits FGFR1 and PDGFR tyrosine kinases with IC50 value of 46, 51 and 100 nM for FGFR1, PDGFRα and PDGFRβ, respectively. [1] E7080 potently inhibits phosphorylation of VEGFR2 (IC50, 0.83 nM) and VEGFR3 (IC50, 0.36 nM) in HUVECs which is stimulated by VEGF and VEGF-C, respectively. [2] A recent study shows that E7080 treatment (both at 1 μM and 10 μM) results in a significant inhibition of cell migration and invasion by inhibiting FGFR and PDGFR signaling. [3] |
|||||||||||
| In vivo | When orally administrated in a H146 xenograft model, E7080 inhibits the growth of H146 tumor at 30 and 100 mg/kg in a dose-dependent manner and leads to tumor regression at 100 mg/kg. Furthermore, E7080 at 100 mg/kg decreases microvessel density more than anti-VEGF antibody and imatinib treatment. [1] E7080 significantly inhibits local tumor growth in a MDA-MB-231 mammary fat pad (m.f.p.) model with RTVs (calculated tumor volume on day 8/tumor volume on day 1) of 0.81, and reduces both angiogenesis and lymphangiogenesis of established metastatic nodules of MDA-MB-231 tumor in the lymph nodes. [2] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
Customer Product Validation
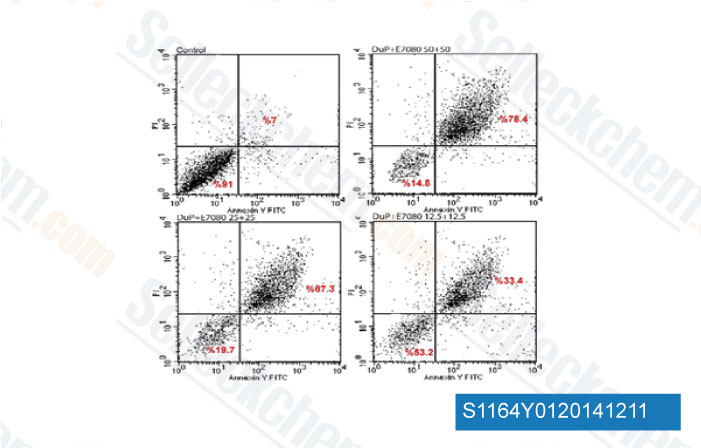
-
Data from [Data independently produced by Asian Pac J Cancer Prev, 2014, 15(7), 3113-21]
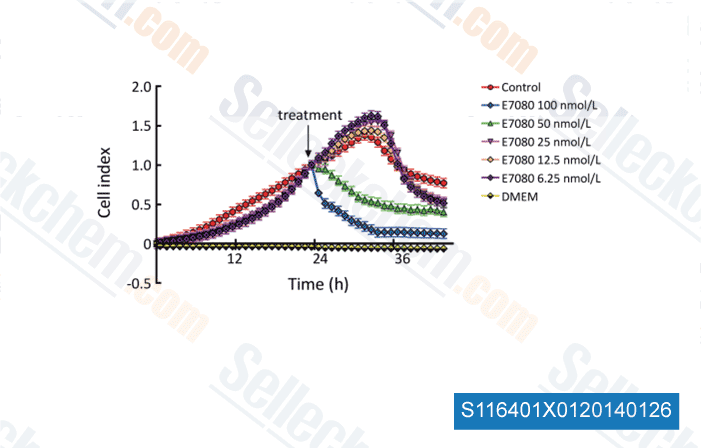
-
Data from [Chin J Cancer Res, 2013, 25(5), 572-84]
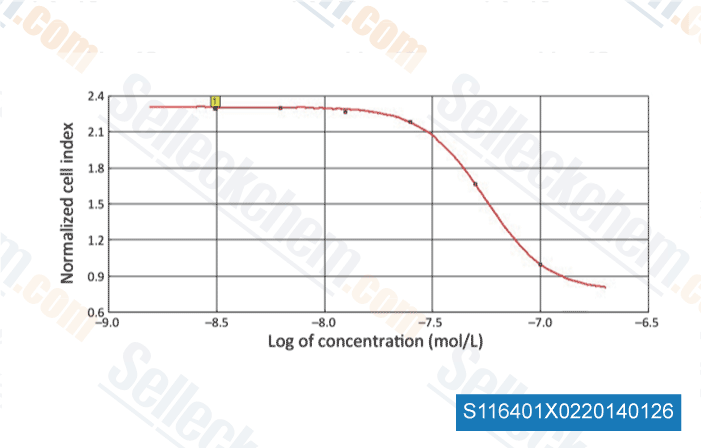
-
Data from [Chin J Cancer Res, 2013, 25(5), 572-84]
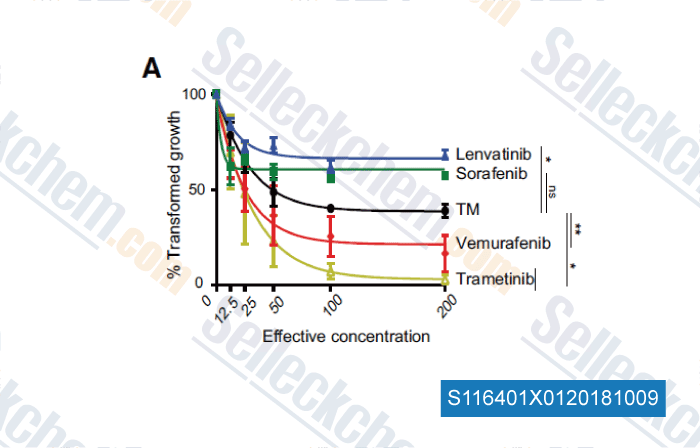
-
Data from [Data independently produced by , , Clin Cancer Res, 2018, 24(17):4271-4281]
Selleck's Lenvatinib has been cited by 113 publications
| Serum amyloid A promotes glycolysis of neutrophils during PD-1 blockade resistance in hepatocellular carcinoma [ Nat Commun, 2024, 15(1):1754] | PubMed: 38409200 |
| Exosomal microRNA profiling revealed enhanced autophagy suppression and anti-tumor effects of a combination of compound Phyllanthus urinaria and lenvatinib in hepatocellular carcinoma [ Phytomedicine, 2024, 10.1016/j.phymed.2023.155091] | PubMed: 37844378 |
| Noncaloric monosaccharides induce excessive sprouting angiogenesis in zebrafish via foxo1a-marcksl1a signal [ bioRxiv, 2024, 10.1101/2024.01.18.576182] | PubMed: none |
| N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells' Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways [ Gastroenterology, 2023, S0016-5085(23)00114-2] | PubMed: 36764493 |
| N6-Methyladenosine-Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells' Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways [ Gastroenterology, 2023, 164(6):990-1005] | PubMed: 36764493 |
| Broad-spectrum kinome profiling identifies CDK6 upregulation as a driver of lenvatinib resistance in hepatocellular carcinoma [ Nat Commun, 2023, 14(1):6699] | PubMed: 37872167 |
| Multiplexed kinase interactome profiling quantifies cellular network activity and plasticity [ Mol Cell, 2023, 83(5):803-818.e8] | PubMed: 36736316 |
| Upregulation of CSNK1A1 induced by ITGB5 confers to hepatocellular carcinoma resistance to sorafenib in vivo by disrupting the EPS15/EGFR complex [ Pharmacol Res, 2023, 192:106789] | PubMed: 37149115 |
| Co-inhibition of glutaminolysis and one-carbon metabolism promotes ROS accumulation leading to enhancement of chemotherapeutic efficacy in anaplastic thyroid cancer [ Cell Death Dis, 2023, 14(8):515] | PubMed: 37573361 |
| Co-inhibition of glutaminolysis and one-carbon metabolism promotes ROS accumulation leading to enhancement of chemotherapeutic efficacy in anaplastic thyroid cancer [ Cell Death Dis, 2023, 14(8):515] | PubMed: 37573361 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
