|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C33H34N6O6 |
|||
| Molecular Weight | 610.66 | CAS No. | 145040-37-5 | |
| Solubility (25°C)* | In vitro | DMSO | 122 mg/mL (199.78 mM) | |
| Ethanol | 4 mg/mL (6.55 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Candesartan Cilexetil (TCV-116) is an angiotensin II receptor antagonist with IC50 of 0.26 nM, used in the treatment of hypertension. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Candesartan blocks the effects of angiotensin II at the angiotensin II type 1 (AT1) receptor. Candesartan cilexetil is a prodrug that is activated to candesartan by ester hydrolysis during gastrointestinal absorption. [1] | ||
| In vivo | Candesartan improves the functional markers in a dose-dependent manner and also upregulates Ang (1-7), ACE2 and mas1 in the myocardium of DCM rats. Candesartan reduces various ER stress and apoptosis markers and the number apoptotic cells in the Candesartan treated rats. [2] Candesartan cilexetil shows angiotensin-II blocking action in a dose-dependent manner in rats with dilated cardiomyopathy. Candesartan cilexetil reduces the left ventricular end-diastolic pressure and heart weight/body weight ratio, the area of myocardial fibrosis and expressions of transforming growth factor-beta1 and collagen-III mRNA. [3] Candesartan cilexetil (1 mg/kg, p.o.) and enalapril (10 mg/kg, p.o.) reduces blood pressure to the same extent 5 hours after administration on the 1st and the 7th day. Candesartan cilexetil significantly increases renal blood flow without any changes in the cardiac index. TCV-116 and enalapril also tends to increase splanchnic blood flow following the 1st dose but not the 7th dose. [4] Candesartan cilexetil is absorbed from the small intestine and hydrolyzed completely to the pharmacologically active metabolite M-I during absorption process. [5] |
Protocol (from reference)
References
Customer Product Validation
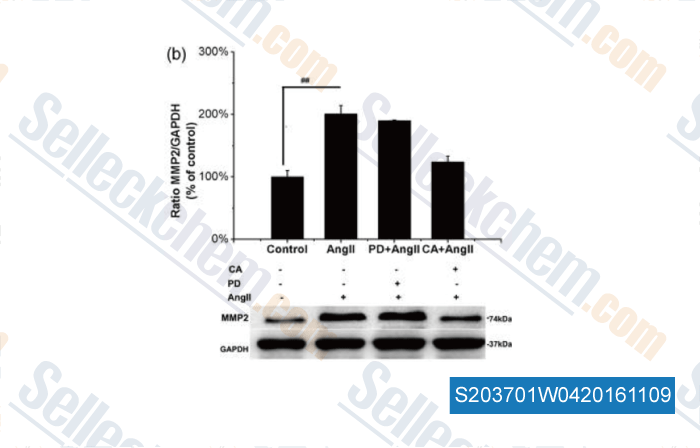
-
, , Exp Biol Med (Maywood), 2015, 240(12):1564-71.
Selleck's Candesartan Cilexetil has been cited by 4 publications
| Candesartan Does Not Activate PPARγ and Its Target Genes in Early Gestation Trophoblasts [ Int J Mol Sci, 2022, 23(20)12326] | PubMed: 36293183 |
| Suppression of adrenal barrestin1-dependent aldosterone production by ARBs: head-to-head comparison [Dabul S, et al. Sci Rep, 2015, 5:8116] | PubMed: 25631300 |
| Angiotensin II increases matrix metalloproteinase 2 expression in human aortic smooth muscle cells via AT1R and ERK1/2. [Wang C, et al. Exp Biol Med, 2015, 10.1177/1535370215576312 ] | PubMed: 25767191 |
| Angiotensin II Induces an Increase in Matrix Metalloproteinase 2 Expression in Aortic Smooth Muscle Cells of Ascending Thoracic Aortic Aneurysms Through JNK, ERK1/2, and p38 MAPK Activation [ J Cardiovasc Pharmacol, 2015, 66(3):285-93] | PubMed: 25955575 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
