|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C18H14F4N2O4S |
|||
| Molecular Weight | 430.37 | CAS No. | 90357-06-5 | |
| Solubility (25°C)* | In vitro | DMSO | 86 mg/mL (199.82 mM) | |
| Ethanol | 4 mg/mL (9.29 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Bicalutamide is an androgen receptor (AR) antagonist with IC50 of 0.16 μM in LNCaP/AR(cs)cell line. Bicalutamide promotes autophagy. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Bicalutamide undergoes an antagonist-to-agonist switch, stimulating AR activity. Bicalutamide treatment of LNCaP/AR(cs) cells in absence of the synthetic androgen R1881 results in altered gene expression consistent with its well-documented agonist activity in context of AR overexpression. Bicalutamide induces cell proliferation in a dose-dependent manner, and only partially antagonized the effects of R1881. Bicalutamide treatment also results in a significant amount of nuclear AR, although less than that observed with R1881. Bicalutamide exhibits partial agonist activity as evidenced by induction of DNA binding at AR target genes and incomplete antagonism of the effects of R1881. In absence of R1881, Bicalutamide partially activates VP16-AR–mediated transcription, indicative of AR binding to DNA. In LNCaP/AR-luc cells with a stably integrates AR-driven luciferase reporter construct. In the presence of R1881, Bicalutamide shows only weak partial antagonism of VP16-AR–mediated transcription with an IC50 of 0.35 μM. [1] Micromolar bicalutamide causes a significant dose-dependent reduction in clonogenicity. [2] Dual inhibition of the AR and mTOR signaling pathways provides further benefit with the ridaforolimus-bicalutamide combination producing syner -gistic antiproliferative effects in prostate cancer cells in vitro when compared with each agent alone. [3] | ||
| In vivo | Single bicalutamide reduces tumor growth by 79%, at defined submaximal doses. The ridaforolimus-bicalutamide combination exhibits improved and potent antitumor activity, almost completely abrogating tumor growth. The combination is also well tolerated, as evidenced by no significant changes in body weight over the course of treatment. Plasma PSA levels are again tightly linked to tumor growth in the combination-treated mice. [3] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[3] |
|
| Animal Study:[3] |
|
References
|
Customer Product Validation
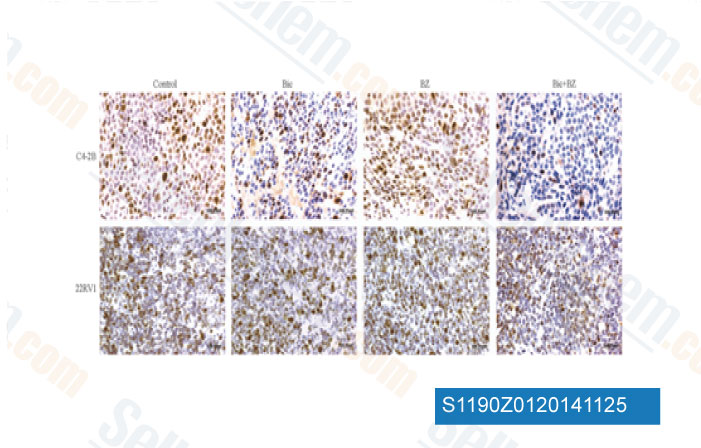
-
Data from [Oncogene, 2014, 10.1038/onc.2014.302]
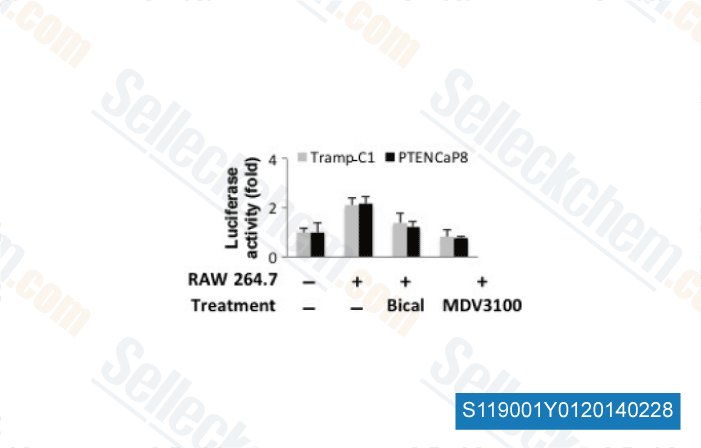
-
Data from [Cancer Sci, 2013, 104(8), 1027-32]
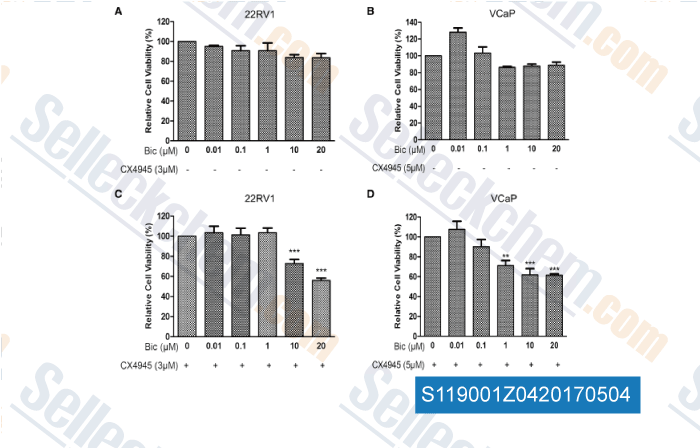
-
, , World J Urol, 2017, 35(8):1213-1221
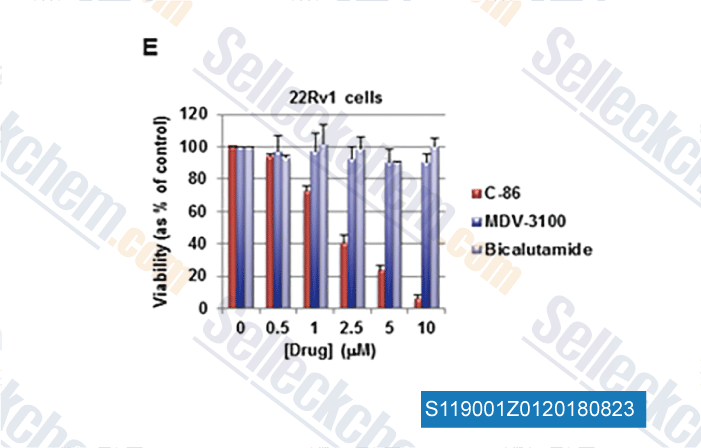
-
Data from [Data independently produced by , , Cancer Res, 2018, doi:10.1158/0008-5472.CAN-17-3728]
Selleck's Bicalutamide has been cited by 55 publications
| GL-V9 inhibits the activation of AR-AKT-HK2 signaling networks and induces prostate cancer cell apoptosis through mitochondria-mediated mechanism [ iScience, 2024, 27(3):109246] | PubMed: 38439974 |
| Gαi2 Protein Inhibition Blocks Chemotherapy- and Anti-Androgen-Induced Prostate Cancer Cell Migration [ Cancers (Basel), 2024, 16(2)296] | PubMed: 38254786 |
| Therapeutic Potential of Bipolar Androgen Therapy for Castration-Resistant Prostate Cancer: In Vitro and In Vivo Studies [ Biomedicines, 2024, 12(1)181] | PubMed: 38255286 |
| AR antagonists develop drug resistance through TOMM20 autophagic degradation-promoted transformation to neuroendocrine prostate cancer [ J Exp Clin Cancer Res, 2023, 42(1):204] | PubMed: 37563661 |
| Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy [ Cell Metab, 2022, S1550-4131-2200411-9] | PubMed: 36257316 |
| Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy [ Theranostics, 2022, 12(11):4965-4979] | PubMed: 35836810 |
| The ERα-NRF2 signalling axis promotes bicalutamide resistance in prostate cancer [ Cell Commun Signal, 2022, 20(1):178] | PubMed: 36376959 |
| CYP1B1-catalyzed 4-OHE2 promotes the castration resistance of prostate cancer stem cells by estrogen receptor α-mediated IL6 activation [ Cell Commun Signal, 2022, 20(1):31] | PubMed: 35292057 |
| α-Terthienyl induces prostate cancer cell death through inhibiting androgen receptor expression [ Biomed Pharmacother, 2022, 152:113266] | PubMed: 35691152 |
| TLK1-mediated MK5-S354 phosphorylation drives prostate cancer cell motility and may signify distinct pathologies [ Mol Oncol, 2022, 16(13):2537-2557] | PubMed: 35064619 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
